Anti-aggregant tau mutant promotes neurogenesis
- PMID: 29202785
- PMCID: PMC5715613
- DOI: 10.1186/s13024-017-0230-8
Anti-aggregant tau mutant promotes neurogenesis
Abstract
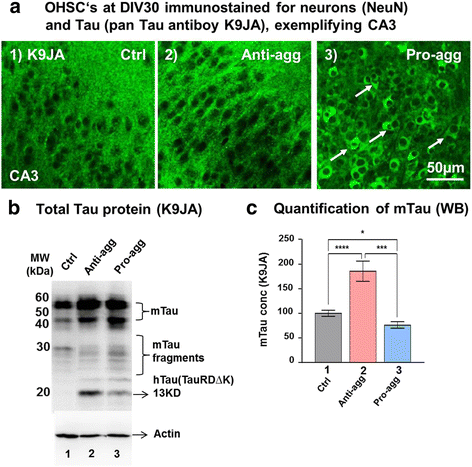
Background: The microtubule-associated protein Tau plays a role in neurodegeneration as well as neurogenesis. Previous work has shown that the expression of the pro-aggregant mutant Tau repeat domain causes strong aggregation and pronounced neuronal loss in the hippocampus whereas the anti-aggregant form has no deleterious effects. These two proteins differ mainly in their propensity to form ß structure and hence to aggregate.
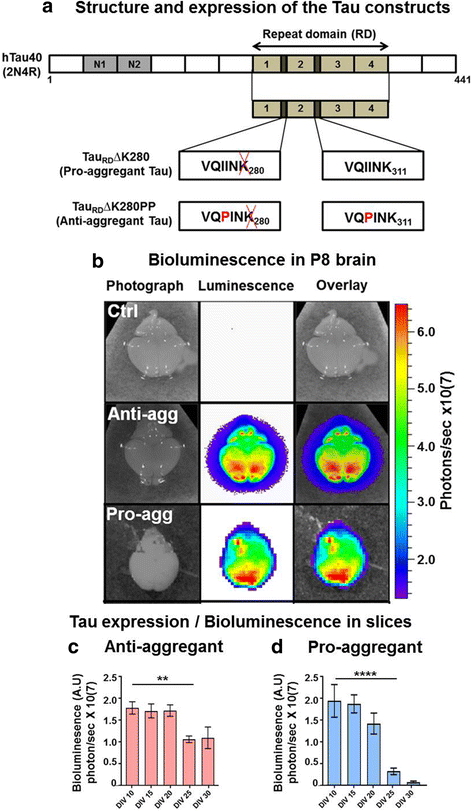
Methods: To elucidate the basis of these contrasting effects, we analyzed organotypic hippocampal slice cultures (OHSCs) from transgenic mice expressing the repeat domain (RD) of Tau with the anti-aggregant mutation (TauRDΔKPP) and compared them with slices containing pro-aggregant TauRDΔK. Transgene expression in the hippocampus was monitored via a sensitive bioluminescence reporter gene assay (luciferase).
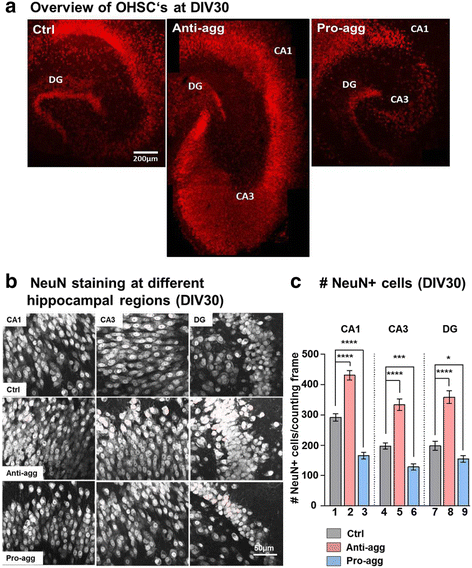
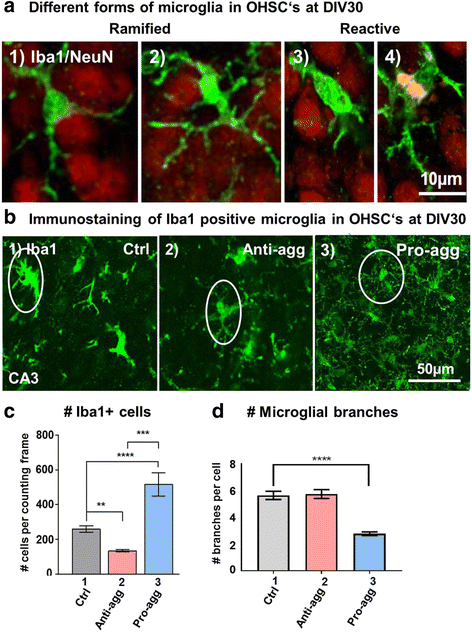
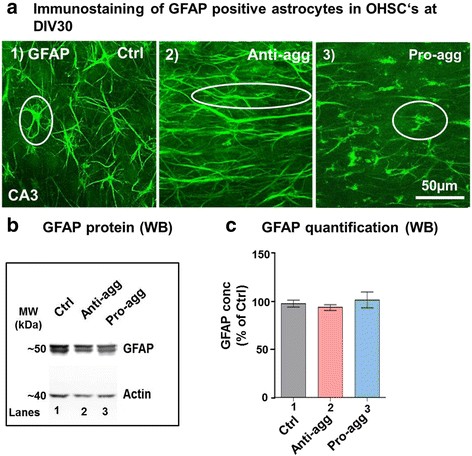
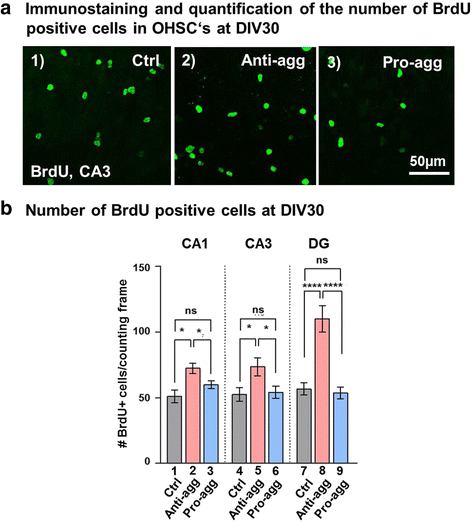
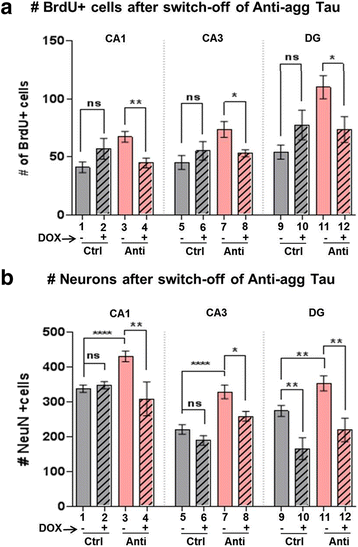
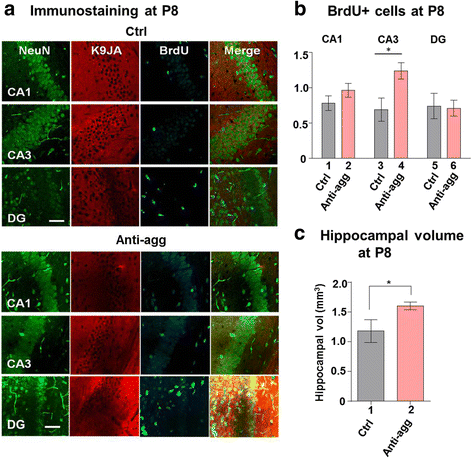
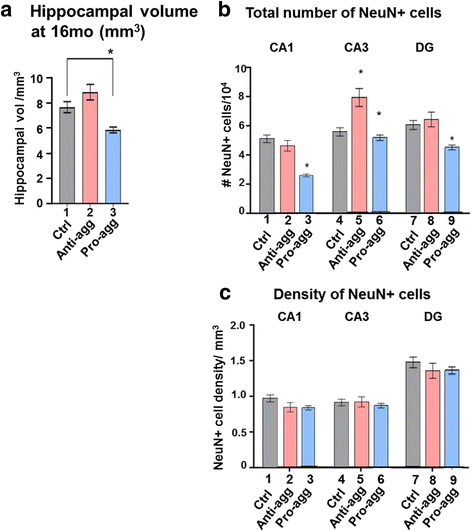
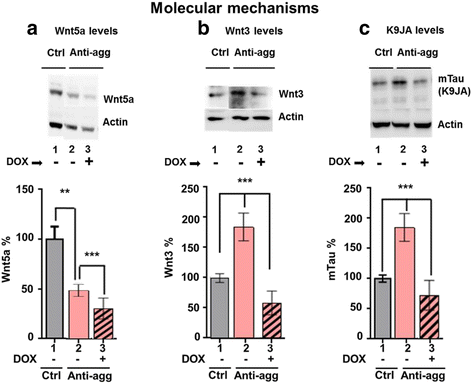
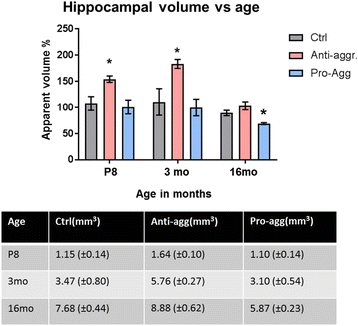
Results: The expression of the anti-aggregant TauRDΔKPP leads to a larger volume of the hippocampus at a young age due to enhanced neurogenesis, resulting in an increase in neuronal number. There were no signs of activation of microglia and astrocytes, indicating the absence of an inflammatory reaction. Investigation of signaling pathways showed that Wnt-5a was strongly decreased whereas Wnt3 was increased. A pronounced increase in hippocampal stem cell proliferation (seen by BrdU) was observed as early as P8, in the CA regions where neurogenesis is normally not observed. The increase in neurons persisted up to 16 months of age.
Conclusion: The data suggest that the expression of anti-aggregant TauRDΔKPP enhances hippocampal neurogenesis mediated by the canonical Wnt signaling pathway, without an inflammatory reaction. This study points to a role of tau in brain development and neurogenesis, in contrast to its detrimental role in neurodegeneration at later age.
Keywords: CA3; Hippocampus; Neurogenesis; Tau; Wnt.
Conflict of interest statement
Ethical approval
Animals were housed and tested according to standards of the German Animal Welfare Act.
Consent for publication
The authors have no conflict of interest.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Apostolova LG, Green AE, Babakchanian S, Hwang KS, Chou YY, Toga AW, Thompson PM. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer Disease. Alzheimer Dis Assoc Disord. 2012;26:17–27. doi: 10.1097/WAD.0b013e3182163b62. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

