PERFECTED enhanced recovery (PERFECT-ER) care versus standard acute care for patients admitted to acute settings with hip fracture identified as experiencing confusion: study protocol for a feasibility cluster randomized controlled trial
- PMID: 29202786
- PMCID: PMC5715500
- DOI: 10.1186/s13063-017-2303-y
PERFECTED enhanced recovery (PERFECT-ER) care versus standard acute care for patients admitted to acute settings with hip fracture identified as experiencing confusion: study protocol for a feasibility cluster randomized controlled trial
Abstract
Background: Health and social care provision for an ageing population is a global priority. Provision for those with dementia and hip fracture has specific and growing importance. Older people who break their hip are recognised as exceptionally vulnerable to experiencing confusion (including but not exclusively, dementia and/or delirium and/or cognitive impairment(s)) before, during or after acute admissions. Older people experiencing hip fracture and confusion risk serious complications, linked to delayed recovery and higher mortality post-operatively. Specific care pathways acknowledging the differences in patient presentation and care needs are proposed to improve clinical and process outcomes.
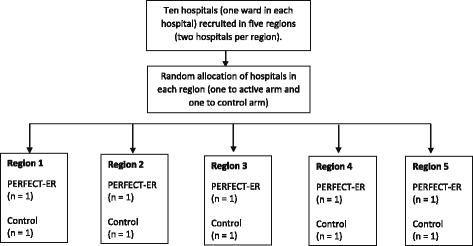
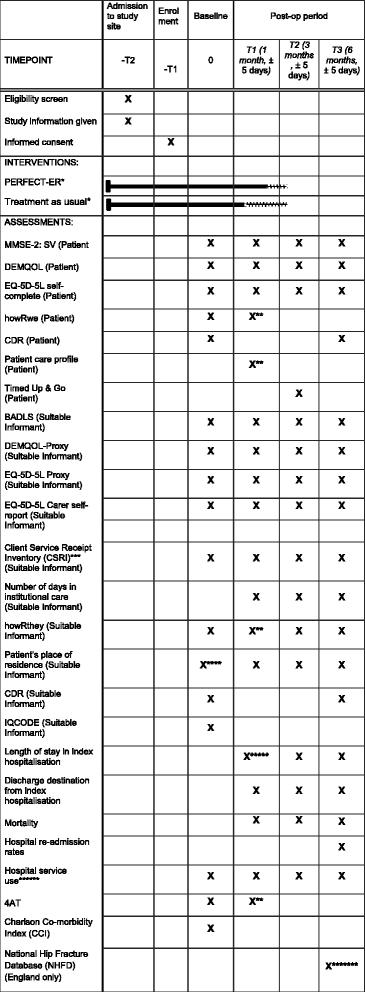
Methods: This protocol describes a multi-centre, feasibility, cluster-randomised, controlled trial (CRCT) to be undertaken across ten National Health Service hospital trusts in the UK. The trial will explore the feasibility of undertaking a CRCT comparing the multicomponent PERFECTED enhanced recovery intervention (PERFECT-ER), which acknowledges the differences in care needs of confused older patients experiencing hip fracture, with standard care. The trial will also have an integrated process evaluation to explore how PERFECT-ER is implemented and interacts with the local context. The study will recruit 400 hip fracture patients identified as experiencing confusion and will also recruit "suitable informants" (individuals in regular contact with participants who will complete proxy measures). We will also recruit NHS professionals for the process evaluation. This mixed methods design will produce data to inform a definitive evaluation of the intervention via a large-scale pragmatic randomised controlled trial (RCT).
Discussion: The trial will provide a preliminary estimate of potential efficacy of PERFECT-ER versus standard care; assess service delivery variation, inform primary and secondary outcome selection, generate estimates of recruitment and retention rates, data collection difficulties, and completeness of outcome data and provide an indication of potential economic benefits. The process evaluation will enhance knowledge of implementation delivery and receipt.
Trial registration: ISRCTN, 99336264 . Registered on 5 September 2016.
Keywords: Acute care; Dementia; Feasibility; Hip fracture; Hospital; Service improvement.
Conflict of interest statement
Ethics approval and consent to participate
Due to the devolved legislative landscape in the UK, ethical approval was sought from separate Research Ethics Committees (REC) in England and Scotland. Ethical approval was granted from Camden and Kings Research Ethics Committee (04.07.2016, reference number: 16/LO/0621) and Scotland Research Ethics Committee A (28.06.2016, reference number: 16/SS/0086) accordingly. Informed consent, or in cases where a patient is adjudged to have lost capacity, consultee agreement (in England) or legal representative consent will be gained from all participants enrolled in the study. As per Health Research Association (HRA), the co-ordinating centre will apply to HRA and REC should protocol amendments be required and, following REC and HRA approval cascade these to research sites. The trial has an independent Trial Steering Committee and Data Monitoring and Ethical Committee. These groups are chaired and composed of independent clinical, academic and service user experts. The membership and terms of reference for these groups are available from the PERFECTED Chief Investigator on request, as is the full version of the protocol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- CG124 - The management of hip fracture in adults. [https://www.nice.org.uk/guidance/cg124/]. Accessed 3 May 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

