Coinfection modulates inflammatory responses, clinical outcome and pathogen load of H1N1 swine influenza virus and Haemophilus parasuis infections in pigs
- PMID: 29202835
- PMCID: PMC5716233
- DOI: 10.1186/s12917-017-1298-7
Coinfection modulates inflammatory responses, clinical outcome and pathogen load of H1N1 swine influenza virus and Haemophilus parasuis infections in pigs
Abstract
Background: Respiratory co-infections are important factor affecting the profitability of pigs production. Swine influenza virus (SIV) may predispose to secondary infection. Haemophilus parasuis (Hps) can be a primary pathogen or be associated with other pathogens such as SIV. To date, little is known about the effect of coinfection with SIV and Hps on the disease severity and inflammatory response and the role of Hps in the induction of pneumonia in the absence of other respiratory pathogens. In the study we investigated the influence of SIV and Hps coinfection on clinical course, inflammatory response, pathogens shedding and load at various time points following intranasal inoculation. The correlation between local concentration of cytokines and severity of disease as well as serum acute phase proteins (APP) concentration has been also studied.
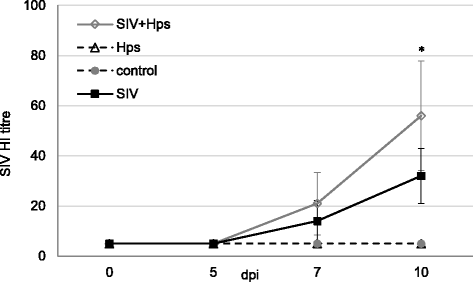
Results: All co-infected pigs had fever, while in single inoculated pigs fever was observed only in part of animals. Necropsy revealed lesions in the lungs all SIV-inoculated and co-inoculated pigs, while in Hps-single inoculated animals only 1 out of 11 pigs revealed gross lung lesions. The SIV shedding was the highest in co-inoculated pigs. There were no differences between Hps-single inoculated and co-inoculated groups with regard to Hps shedding. The significant increase in Hps titre in the lung has been found only in co-inoculated group. All APP increased after co-infection. In single-inoculated animals various kinetics of APP response has been observed. The lung concentrations of cytokines were induced mostly in SIV + Hps pigs in the apical and middle lobe. These results correlated well with localization of gross lung lesions.
Conclusions: The results revealed that SIV increased the severity of lung lesions and facilitated Hps (PIWetHps192/2015) replication in the porcine lung. Furthermore, Hps influenced the SIV nasal shedding. Enhanced Hps and SIV replication, together with stronger systemic and local inflammatory response contributed to a more severe clinical signs and stronger, earlier immune response in co-inoculated animals. We confirmed the previous evidence that single-Hps infection does not produce significant pneumonic lesions but it should be in mind that other strains of Hps may produce lesions different from that reported in the present study.
Keywords: Disease severity; Immunity; Pathogens shedding; Pigs; Respiratory co-infections.
Conflict of interest statement
Ethics approval and consent to participate
Animal use and handling protocols were approved by II Local Ethical Commission for the Animal Experiments of University of Life Sciences in Lublin (number of approval: 77/2014).
Consent for publication
Not applicable.
Competing interests
None of the authors of this paper has a relationship with other people or organisations that could influence or bias the content of the paper. The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures










Similar articles
-
Kinetics of single and dual infection of pigs with swine influenza virus and Actinobacillus pleuropneumoniae.Vet Microbiol. 2017 Mar;201:113-120. doi: 10.1016/j.vetmic.2017.01.011. Epub 2017 Jan 17. Vet Microbiol. 2017. PMID: 28284596
-
Pre-infection of pigs with Mycoplasma hyopneumoniae modifies outcomes of infection with European swine influenza virus of H1N1, but not H1N2, subtype.Vet Microbiol. 2012 May 25;157(1-2):96-105. doi: 10.1016/j.vetmic.2011.12.027. Epub 2011 Dec 29. Vet Microbiol. 2012. PMID: 22261237 Free PMC article.
-
Concurrent highly pathogenic porcine reproductive and respiratory syndrome virus infection accelerates Haemophilus parasuis infection in conventional pigs.Vet Microbiol. 2012 Aug 17;158(3-4):316-21. doi: 10.1016/j.vetmic.2012.03.001. Epub 2012 Mar 8. Vet Microbiol. 2012. PMID: 22460022
-
[Swine influenza virus: evolution mechanism and epidemic characterization--a review].Wei Sheng Wu Xue Bao. 2009 Sep;49(9):1138-45. Wei Sheng Wu Xue Bao. 2009. PMID: 20030049 Review. Chinese.
-
Fatal case of swine influenza virus in an immunocompetent host.Mayo Clin Proc. 1998 Mar;73(3):243-5. doi: 10.4065/73.3.243. Mayo Clin Proc. 1998. PMID: 9511782 Review.
Cited by
-
Identification of Glaesserella parasuis and Differentiation of Its 15 Serovars Using High-Resolution Melting Assays.Pathogens. 2022 Jul 1;11(7):752. doi: 10.3390/pathogens11070752. Pathogens. 2022. PMID: 35889997 Free PMC article.
-
Secondary Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (HP-PRRSV2) Infection Augments Inflammatory Responses, Clinical Outcomes, and Pathogen Load in Glaesserella-parasuis-Infected Piglets.Vet Sci. 2023 May 20;10(5):365. doi: 10.3390/vetsci10050365. Vet Sci. 2023. PMID: 37235448 Free PMC article.
-
Influenza A Virus Detection at the Human-Swine Interface in US Midwest Swine Farms.Viruses. 2024 Dec 15;16(12):1921. doi: 10.3390/v16121921. Viruses. 2024. PMID: 39772228 Free PMC article.
-
Viral and Bacterial Profiles in Endemic Influenza A Virus Infected Swine Herds Using Nanopore Metagenomic Sequencing on Tracheobronchial Swabs.Microbiol Spectr. 2023 Feb 28;11(2):e0009823. doi: 10.1128/spectrum.00098-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 36853049 Free PMC article.
-
Systematic review of animal-based indicators to measure thermal, social, and immune-related stress in pigs.PLoS One. 2022 May 5;17(5):e0266524. doi: 10.1371/journal.pone.0266524. eCollection 2022. PLoS One. 2022. PMID: 35511825 Free PMC article.
References
-
- Truszczyński M, Pejsak Z. Contribution of Pasteurella multocida to the porcine respiratory disease complex. Medycyna Weterynaryjna. 2010;66(8):512–515.
-
- de la Fuente AJ M, Gutierrez Martin CB, Perez Martinez C, Garcia Iglesias MJ, Tejerina F, Rodriguez Ferri EF. Effect of different vaccine formulations on the development of Glasser's disease induced in pigs by experimental Haemophilus parasuis infection. J Comp Pathol. 2009;140(2–3):169–176. doi: 10.1016/j.jcpa.2008.10.007. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

