Nutrition education for cardiovascular disease prevention in individuals with spinal cord injuries: study protocol for a randomized controlled trial
- PMID: 29202852
- PMCID: PMC5716386
- DOI: 10.1186/s13063-017-2263-2
Nutrition education for cardiovascular disease prevention in individuals with spinal cord injuries: study protocol for a randomized controlled trial
Abstract
Background: Individuals with chronic spinal cord injuries (SCIs) have an increased prevalence of cardiovascular disease (CVD) and associated risk factors compared with age-matched control subjects. Exercise has been shown to improve selected CVD risk factors in individuals with SCI, but using nutrition education as an intervention has not been evaluated in this population. This paper describes our research plan for evaluating the effect of nutrition education on individuals with SCI. In the present study, called Eat Smart, Live Better, we are using a randomized controlled design to test an intervention adapted from an existing evidence-based program that showed a positive effect on nutrition knowledge and behavior of older adults from the general population. There will be an inpatient group (n = 100) and a community group (n = 100). The aims of our study are to compare the intervention and control groups for (1) changes in nutritional behavior, nutritional knowledge, and dietary quality by participants in the program; (2) levels of adiposity and metabolic CVD risk factors at 12-month follow-up; and (3) differential effects among individuals with SCI in the acute rehabilitation setting and those living in the community.
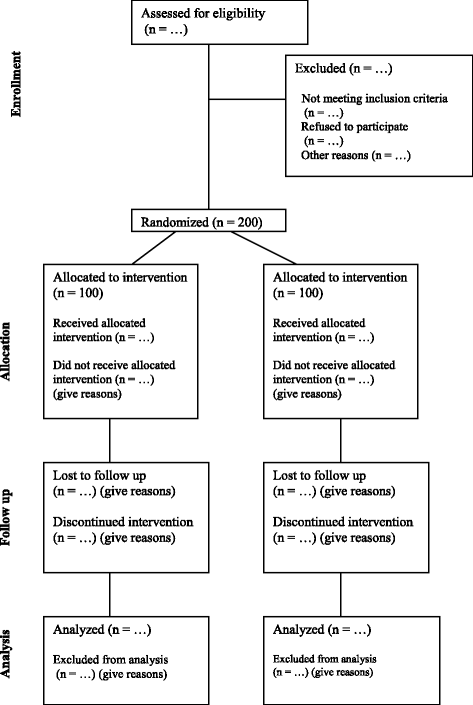
Methods/design: This is a randomized controlled trial of nutrition education. The treatment groups receive six nutrition education sessions. The control groups receive the one "standard of care" nutrition lecture that is required by the Commission on Accreditation of Rehabilitation Facilities. Treatment groups include both an inpatient group, comprising patients who have been admitted to an acute rehabilitation facility because of their recent SCI, and an outpatient group, consisting of community-dwelling adults who are at least 1 year after their SCI. A total of 200 participants will be randomized 1:1 to the intervention or control group, stratified by location (acute rehabilitation facility or community dwelling).
Discussion: To our knowledge, this will be the first reported study of nutrition education in individuals with SCI. The low cost and feasibility of the intervention, if shown to improve nutritional behavior, suggests that it could be implemented in rehabilitation facilities across the country. This has the potential of lowering the burden of CVD and CVD risk factors in this high-risk population.
Trial registration: ClinicalTrials.gov, NCT02368405 . Registered on February 10, 2015.
Keywords: Cardiovascular disease prevention; Nutrition education; Spinal cord injury.
Conflict of interest statement
Ethics approval and consent to participate
This study has received IRB approval from the Carolinas Healthcare System IRB (reference number 09-14-06E). Any important protocol modifications will be sent to the IRB for approval. All participants will go through the informed consent process.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. There will not be any professional writers used.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E. Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr. 1998;68(6):1223–1227. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

