Neuronal CCL2 expression drives inflammatory monocyte infiltration into the brain during acute virus infection
- PMID: 29202854
- PMCID: PMC5715496
- DOI: 10.1186/s12974-017-1015-2
Neuronal CCL2 expression drives inflammatory monocyte infiltration into the brain during acute virus infection
Abstract
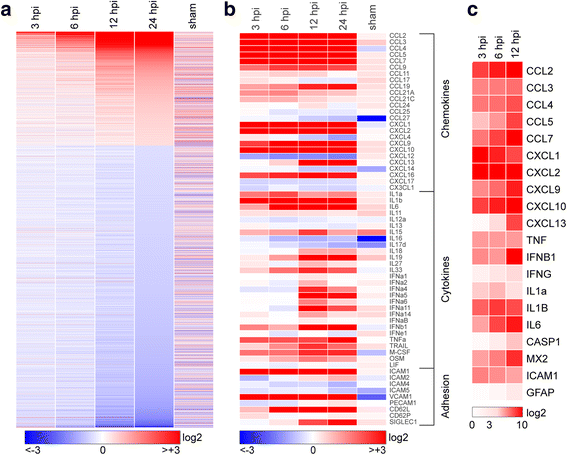
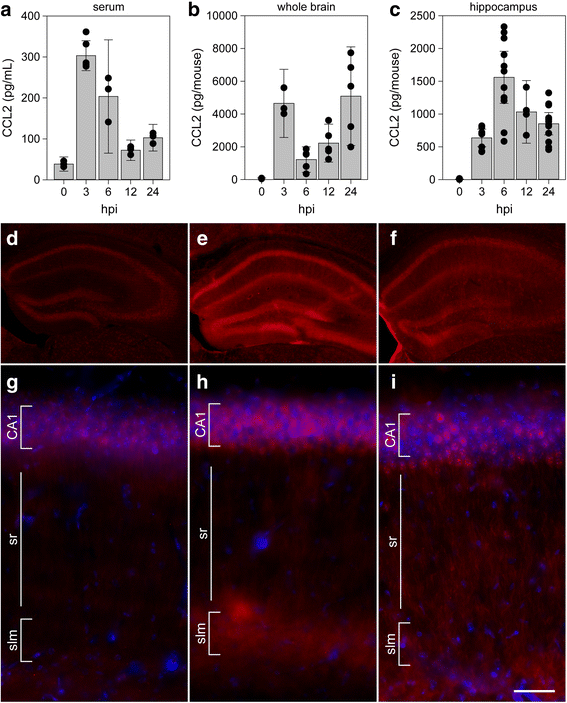
Background: Viral encephalitis is a dangerous compromise between the need to robustly clear pathogen from the brain and the need to protect neurons from bystander injury. Theiler's murine encephalomyelitis virus (TMEV) infection of C57Bl/6 mice is a model of viral encephalitis in which the compromise results in hippocampal damage and permanent neurological sequelae. We previously identified brain-infiltrating inflammatory monocytes as the primary driver of this hippocampal pathology, but the mechanisms involved in recruiting these cells to the brain were unclear.
Methods: Chemokine expression levels in the hippocampus were assessed by microarray, ELISA, RT-PCR, and immunofluorescence. Monocyte infiltration during acute TMEV infection was measured by flow cytometry. CCL2 levels were manipulated by immunodepletion and by specific removal from neurons in mice generated by crossing a line expressing the Cre recombinase behind the synapsin promoter to animals with floxed CCL2.
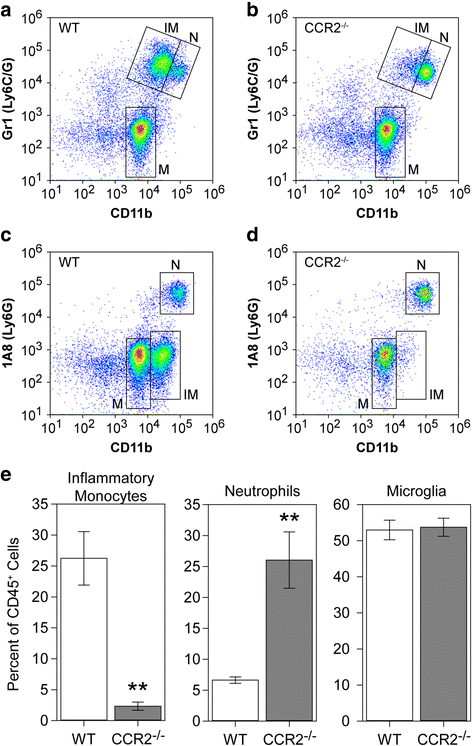
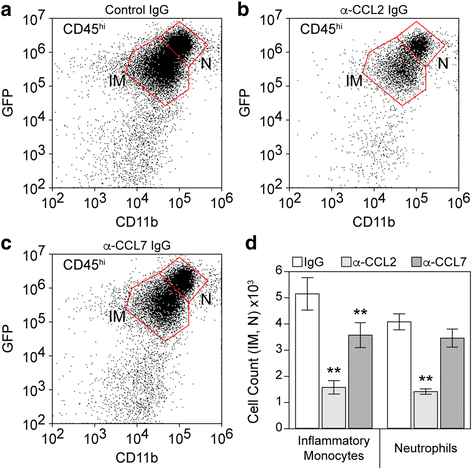
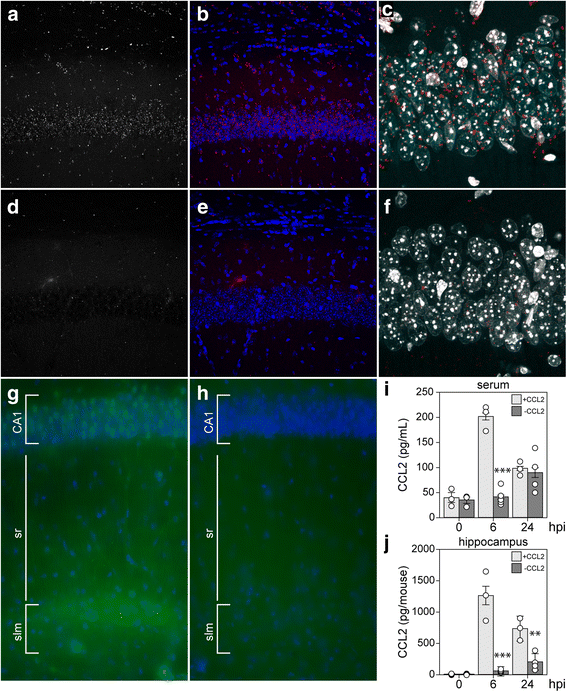
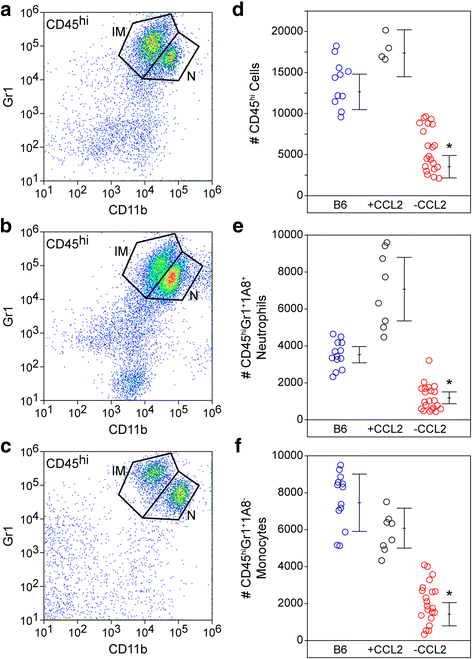
Results: Inoculation of the brain with TMEV induced hippocampal production of the proinflammatory chemokine CCL2 that peaked at 6 h postinfection, whereas inoculation with UV-inactivated TMEV did not elicit this response. Immunofluorescence revealed that hippocampal neurons expressed high levels of CCL2 at this timepoint. Genetic deletion of CCR2 and systemic immunodepletion of CCL2 abrogated or blunted the infiltration of inflammatory monocytes into the brain during acute infection. Specific genetic deletion of CCL2 from neurons reduced serum and hippocampal CCL2 levels and inhibited inflammatory monocyte infiltration into the brain.
Conclusions: We conclude that intracranial inoculation with infectious TMEV rapidly induces the expression of CCL2 in neurons, and this cellular source is necessary for CCR2-dependent infiltration of inflammatory monocytes into the brain during the most acute stage of encephalitis. These findings highlight a unique role for neuronal production of chemokines in the initiation of leukocytic infiltration into the infected central nervous system.
Keywords: CCL2; CCR2; Encephalitis; Hippocampus; Inflammatory monocyte; Theiler’s murine encephalomyelitis virus.
Conflict of interest statement
Ethics approval
All animal experiments were performed according to the National Institutes of Health guidelines and were approved by the Mayo Clinic Institutional Animal Care and Use Committee (Animal Welfare Assurance number A3291-01).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Inflammatory monocytes damage the hippocampus during acute picornavirus infection of the brain.J Neuroinflammation. 2012 Mar 9;9:50. doi: 10.1186/1742-2094-9-50. J Neuroinflammation. 2012. PMID: 22405261 Free PMC article.
-
Macrophage depletion by liposome-encapsulated clodronate suppresses seizures but not hippocampal damage after acute viral encephalitis.Neurobiol Dis. 2018 Feb;110:192-205. doi: 10.1016/j.nbd.2017.12.001. Epub 2017 Dec 5. Neurobiol Dis. 2018. PMID: 29208406
-
Chemokine receptors CCR2 and CX3CR1 regulate viral encephalitis-induced hippocampal damage but not seizures.Proc Natl Acad Sci U S A. 2018 Sep 18;115(38):E8929-E8938. doi: 10.1073/pnas.1806754115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181265 Free PMC article.
-
Differential usage of carbohydrate co-receptors influences cellular tropism of Theiler's murine encephalomyelitis virus infection of the central nervous system.Glycoconj J. 2006 Feb;23(1-2):39-49. doi: 10.1007/s10719-006-5436-x. Glycoconj J. 2006. PMID: 16575521 Review.
-
Inflammatory monocytes and the pathogenesis of viral encephalitis.J Neuroinflammation. 2012 Dec 17;9:270. doi: 10.1186/1742-2094-9-270. J Neuroinflammation. 2012. PMID: 23244217 Free PMC article. Review.
Cited by
-
Deficiency in astrocyte CCL2 production reduces neuroimmune control of Toxoplasma gondii infection.PLoS Pathog. 2024 Jan 11;20(1):e1011710. doi: 10.1371/journal.ppat.1011710. eCollection 2024 Jan. PLoS Pathog. 2024. PMID: 38206985 Free PMC article.
-
Inflammatory responses to a pathogenic West Nile virus strain.BMC Infect Dis. 2019 Oct 29;19(1):912. doi: 10.1186/s12879-019-4471-8. BMC Infect Dis. 2019. PMID: 31664929 Free PMC article.
-
Monocyte-related cytokines/chemokines in cerebral ischemic stroke.CNS Neurosci Ther. 2023 Dec;29(12):3693-3712. doi: 10.1111/cns.14368. Epub 2023 Jul 14. CNS Neurosci Ther. 2023. PMID: 37452512 Free PMC article. Review.
-
Psychological stress induces depressive-like behavior associated with bone marrow-derived monocyte infiltration into the hippocampus independent of blood-brain barrier disruption.J Neuroinflammation. 2022 Aug 24;19(1):208. doi: 10.1186/s12974-022-02569-w. J Neuroinflammation. 2022. PMID: 36002834 Free PMC article.
-
Inflammatory monocytes and microglia play independent roles in inflammatory ictogenesis.J Neuroinflammation. 2022 Jan 29;19(1):22. doi: 10.1186/s12974-022-02394-1. J Neuroinflammation. 2022. PMID: 35093106 Free PMC article.
References
-
- Umpierre AD, Remigio GJ, Dahle EJ, Bradford K, Alex AB, Smith MD, West PJ, White HS, Wilcox KS. Impaired cognitive ability and anxiety-like behavior following acute seizures in the Theiler’s virus model of temporal lobe epilepsy. Neurobiol Dis. 2014;64:98–106. doi: 10.1016/j.nbd.2013.12.015. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

