Testing the 4Rs and 2Ss Multiple Family Group intervention: study protocol for a randomized controlled trial
- PMID: 29202867
- PMCID: PMC5716003
- DOI: 10.1186/s13063-017-2331-7
Testing the 4Rs and 2Ss Multiple Family Group intervention: study protocol for a randomized controlled trial
Abstract
Background: Oppositional defiant disorder (ODD) is a major mental health concern and highly prevalent among children living in poverty-impacted communities. Despite that treatments for ODD are among the most effective, few children living in poverty receive these services due to substantial barriers to access, as well as difficulties in the uptake and sustained adoption of evidence-based practices (EBPs) in community settings. The purpose of this study is to examine implementation processes that impact uptake of an evidence-based practice for childhood ODD, and the impact of a Clinic Implementation Team (CIT)-driven structured adaptation to enhance its fit within the public mental health clinic setting.
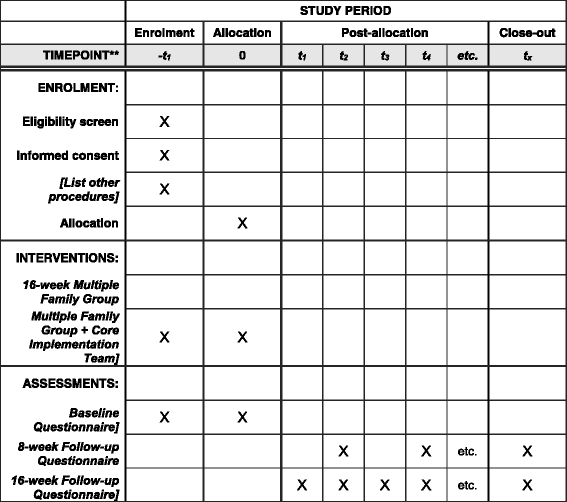
Methods/design: This study, a Hybrid Type II effectiveness-implementation research trial, blends clinical effectiveness and implementation research methods to examine the impact of the 4Rs and 2Ss Multiple Family Group (MFG) intervention, family level mediators of child outcomes, clinic/provider-level mediators of implementation, and the impact of CITs on uptake and long-term utilization of this model. All New York City public outpatient mental health clinics have been invited to participate. A sampling procedure that included randomization at the agency level and a sub-study to examine the impact of clinic choice upon outcomes yielded a distribution of clinics across three study conditions. Quantitative data measuring child outcomes, organizational factors and implementation fidelity will be collected from caregivers and providers at baseline, 8, and 16 weeks from baseline, and 6 months from treatment completion. The expected participation is 134 clinics, 268 providers, and 2688 caregiver/child dyads. We will use mediation analysis with a multi-level Structural Equation Modeling (SEM) (MSEM including family level variables, provider variables, and clinic variables), as well as mediation tests to examine study hypotheses.
Discussion: The aim of the study is to generate knowledge about effectiveness and mediating factors in the treatment of ODDs in children in the context of family functioning, and to propose an innovative approach to the adaptation and implementation of new treatment interventions within clinic settings. The proposed CIT adaptation and implementation model has the potential to enhance implementation and sustainability, and ultimately increase the extent to which effective interventions are available and can impact children and families in need of services for serious behavior problems.
Trial registration: ClinicalTrials.gov, ID: NCT02715414 . Registered on 3 March 2016.
Keywords: Child mental health; Family functioning; Implementation and sustainability; Oppositional defiant disorder.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by New York University’s Institutional Review Board (IRB). The study was also approved by agencies’ own IRBs when applicable (a list of all ethics committees is attached). An explanation of the study procedures and its risks and benefits is provided to all caregivers and providers via an Informed Consent Form. Participants have an opportunity to read and to receive clarification from the research staff on the content of the Informed Consent Form as well as answers to any additional questions or concerns they might have. Participants receive a copy of the Consent Form, which contains the principal investigator’s contact information and the contact information for the IRB that approved the research project (New York University IRB, number 14-10423). Children are required to give verbal assent in order to participate in the group and to have their caregivers answer questions about them. Research personnel explain the study to the children, using age-appropriate language to ensure their understanding. Children are given the option not to participate, and are assured that refusal to participate will not affect the services that they receive.
All letters of approval from IRBs are attached as a separate document. The New York University IRB was used when agencies did not have their own ethics review board. In the circumstance that agencies had their own IRB, additional approval was sought from their agency as well. The protocol was reviewed by the Services Research and Clinical Epidemiology Branch at the National Institute of Mental Health.
Consent for publication
Any results that will be published in academic journals will adhere to New York University’s IRB guidelines.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Strengthening mental health and research training in Sub-Saharan Africa (SMART Africa): Uganda study protocol.Trials. 2018 Aug 6;19(1):423. doi: 10.1186/s13063-018-2751-z. Trials. 2018. PMID: 30081967 Free PMC article.
-
Implementing an early childhood school-based mental health promotion intervention in low-resource Ugandan schools: study protocol for a cluster randomized controlled trial.Trials. 2014 Dec 1;15:471. doi: 10.1186/1745-6215-15-471. Trials. 2014. PMID: 25443043 Free PMC article. Clinical Trial.
-
Cornerstone program for transition-age youth with serious mental illness: study protocol for a randomized controlled trial.Trials. 2016 Nov 8;17(1):537. doi: 10.1186/s13063-016-1654-0. Trials. 2016. PMID: 27825381 Free PMC article. Clinical Trial.
-
Behavioral family interventions for improving child-rearing: a review of the literature for clinicians and policy makers.Clin Child Fam Psychol Rev. 1998 Mar;1(1):41-60. doi: 10.1023/a:1021848315541. Clin Child Fam Psychol Rev. 1998. PMID: 11324077 Review.
-
Potential Mediators in Parenting and Family Intervention: Quality of Mediation Analyses.Clin Child Fam Psychol Rev. 2017 Jun;20(2):127-145. doi: 10.1007/s10567-016-0221-2. Clin Child Fam Psychol Rev. 2017. PMID: 28028654 Free PMC article. Review.
Cited by
-
Perceived Benefits of a Multiple Family Group for Children with Behavior Problems and their Families.Soc Work Groups. 2019;42(3):197-212. doi: 10.1080/01609513.2019.1567437. Epub 2019 Feb 4. Soc Work Groups. 2019. PMID: 31827309 Free PMC article.
-
Providers' Perspectives on Implementing a Multiple Family Group for Children with Disruptive Behavior.J Child Fam Stud. 2020 Apr;29(4):1008-1020. doi: 10.1007/s10826-019-01667-3. Epub 2019 Dec 23. J Child Fam Stud. 2020. PMID: 33343177 Free PMC article.
-
Examining Organizational Factors Supporting the Adoption and Use of Evidence-Based Interventions.Community Ment Health J. 2021 Aug;57(6):1187-1194. doi: 10.1007/s10597-020-00751-z. Epub 2021 Jan 2. Community Ment Health J. 2021. PMID: 33387179 Clinical Trial.
-
Outcomes Associated With Adapting a Research-Supported Treatment for Children With Behavior Disorders.Res Soc Work Pract. 2020 Jan;30(1):74-83. doi: 10.1177/1049731519841439. Epub 2019 May 1. Res Soc Work Pract. 2020. PMID: 32855587 Free PMC article.
-
An Examination of the 4 Rs 2 Ss for Problem Behaviors: A Preventive Approach.Fam Soc. 2023 Jun;104(2):154-166. doi: 10.1177/10443894221133419. Epub 2022 Dec 20. Fam Soc. 2023. PMID: 37408541 Free PMC article.
References
-
- Acri M, Gopalan G, Chacko A, McKay M. (in press). Engaging families into treatment for child behavior disorders: a synthesis of the literature. In: Lochman JE, Matthys W, editors. The Wiley handbook of disruptive and impulse-control disorders. New York: Wiley.
-
- National Institute of Child, Health and Human Development Early Child Care Research Network. Duration and developmental timing of poverty and children’s cognitive and social development from birth through third grade. Child Dev. 2005;76(4):795–810. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical