Cellular Concentrations of the Transporters DctA and DcuB and the Sensor DcuS of Escherichia coli and the Contributions of Free and Complexed DcuS to Transcriptional Regulation by DcuR
- PMID: 29203472
- PMCID: PMC5786699
- DOI: 10.1128/JB.00612-17
Cellular Concentrations of the Transporters DctA and DcuB and the Sensor DcuS of Escherichia coli and the Contributions of Free and Complexed DcuS to Transcriptional Regulation by DcuR
Abstract
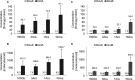
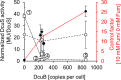
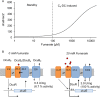
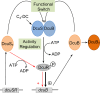
In Escherichia coli, the catabolism of C4-dicarboxylates is regulated by the DcuS-DcuR two-component system. The functional state of the sensor kinase DcuS is controlled by C4-dicarboxylates (like fumarate) and complexation with the C4-dicarboxylate transporters DctA and DcuB, respectively. Free DcuS (DcuSF) is known to be constantly active even in the absence of fumarate, whereas the DcuB-DcuS and DctA-DcuS complexes require fumarate for activation. To elucidate the impact of the transporters on the functional state of DcuS and the concentrations of DcuSF and DcuB-DcuS (or DctA-DcuS), the absolute levels of DcuS, DcuB, and DctA were determined in aerobically or anaerobically grown cells by mass spectrometry. DcuS was present at a constant very low level (10 to 20 molecules DcuS/cell), whereas the levels of DcuB and DctA were higher (minimum, 200 molecules/cell) and further increased with fumarate (12.7- and 2.7-fold, respectively). Relating DcuS and DcuB contents with the functional state of DcuS allowed an estimation of the proportions of DcuS in the free (DcuSF) and the complexed (DcuB-DcuS) states. Unexpectedly, DcuSF levels were always low (<2% of total DcuS), ruling out earlier models that show DcuSF as the major species under noninducing conditions. In the absence of fumarate, when DcuSF is responsible for basal dcuB expression, up to 8% of the maximal DcuB levels are formed. These suffice for DcuB-DcuS complex formation and basal transport activity. In the presence of fumarate (>100 μM), the DcuB-DcuS complex drives the majority of dcuB expression and is thus responsible for induction.IMPORTANCE Two-component systems (TCS) are major devices for sensing by bacteria and adaptation to environmental cues. Membrane-bound sensor kinases of TCS often use accessory proteins of unknown function. The DcuS-DcuR TCS responds to C4-dicarboxylates and requires formation of the complex of DcuS with C4-dicarboxylate transporters DctA or DcuB. Free DcuS (DcuSF) is constitutively active in autophosphorylation and was supposed to have a major role under specific conditions. Here, absolute concentrations of DcuS, DcuB, and DctA were determined under activating and nonactivating conditions by mass spectrometry. The relationship of their absolute contents to the functional state of DcuS revealed their contribution to the control of DcuS-DcuR in vivo, which was not accessible by other approaches, leading to a revision of previous models.
Keywords: C4-dicarboxylate sensing; DctA; DcuB; DcuS; Escherichia coli; SRM; absolute quantification; sensor complex.
Copyright © 2018 American Society for Microbiology.
Figures


References
-
- Miles JS, Guest JR. 1987. Molecular genetic aspects of the citric acid cycle of Escherichia coli, p 45–65. In Kay J, Weitzman PDJ (ed), Krebs citric acid cycle. The Biochemical Society, London, United Kingdom. - PubMed
-
- Kröger A, Geisler V, Lemma E, Theis F, Lenger R. 1992. Bacterial fumarate respiration. Arch Microbiol 158:311–314. doi:10.1007/BF00245358. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

