Extreme Drug Tolerance of Mycobacterium tuberculosis in Caseum
- PMID: 29203492
- PMCID: PMC5786764
- DOI: 10.1128/AAC.02266-17
Extreme Drug Tolerance of Mycobacterium tuberculosis in Caseum
Abstract
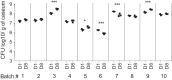
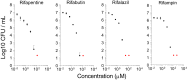
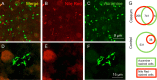
Tuberculosis (TB) recently became the leading infectious cause of death in adults, while attempts to shorten therapy have largely failed. Dormancy, persistence, and drug tolerance are among the factors driving the long therapy duration. Assays to measure in situ drug susceptibility of Mycobacterium tuberculosis bacteria in pulmonary lesions are needed if we are to discover new fast-acting regimens and address the global TB threat. Here we take a first step toward this goal and describe an ex vivo assay developed to measure the cidal activity of anti-TB drugs against M. tuberculosis bacilli present in cavity caseum obtained from rabbits with active TB. We show that caseum M. tuberculosis bacilli are largely nonreplicating, maintain viability over the course of the assay, and exhibit extreme tolerance to many first- and second-line TB drugs. Among the drugs tested, only the rifamycins fully sterilized caseum. A similar trend of phenotypic drug resistance was observed in the hypoxia- and starvation-induced nonreplicating models, but with notable qualitative and quantitative differences: (i) caseum M. tuberculosis exhibits higher drug tolerance than nonreplicating M. tuberculosis in the Wayne and Loebel models, and (ii) pyrazinamide is cidal in caseum but has no detectable activity in these classic nonreplicating assays. Thus, ex vivo caseum constitutes a unique tool to evaluate drug potency against slowly replicating or nonreplicating bacilli in their native caseous environment. Intracaseum cidal concentrations can now be related to the concentrations achieved in the necrotic foci of granulomas and cavities to establish correlations between clinical outcome and lesion-centered pharmacokinetics-pharmacodynamics (PK-PD) parameters.
Keywords: Mycobacterium tuberculosis; caseum; drug tolerance; in vitro potency model; persistence; pharmacokinetics-pharmacodynamics.
Copyright © 2018 Sarathy et al.
Figures

Similar articles
-
A Novel Tool to Identify Bactericidal Compounds against Vulnerable Targets in Drug-Tolerant M. tuberculosis found in Caseum.mBio. 2023 Apr 25;14(2):e0059823. doi: 10.1128/mbio.00598-23. Epub 2023 Apr 5. mBio. 2023. PMID: 37017524 Free PMC article.
-
A Physiologically Relevant In Vitro Model of Nonreplicating Persistent Mycobacterium tuberculosis in Caseum.Curr Protoc. 2025 Mar;5(3):e70118. doi: 10.1002/cpz1.70118. Curr Protoc. 2025. PMID: 40056090 Free PMC article.
-
Role of DNA Double-Strand Break Formation in Gyrase Inhibitor-Mediated Killing of Nonreplicating Persistent Mycobacterium tuberculosis in Caseum.ACS Infect Dis. 2024 Oct 11;10(10):3631-3639. doi: 10.1021/acsinfecdis.4c00499. Epub 2024 Sep 24. ACS Infect Dis. 2024. PMID: 39315541 Free PMC article.
-
Fighting tuberculosis by drugs targeting nonreplicating Mycobacterium tuberculosis bacilli.Int J Mycobacteriol. 2017 Jul-Sep;6(3):213-221. doi: 10.4103/ijmy.ijmy_85_17. Int J Mycobacteriol. 2017. PMID: 28776518 Review.
-
Caseum: a Niche for Mycobacterium tuberculosis Drug-Tolerant Persisters.Clin Microbiol Rev. 2020 Apr 1;33(3):e00159-19. doi: 10.1128/CMR.00159-19. Print 2020 Jun 17. Clin Microbiol Rev. 2020. PMID: 32238365 Free PMC article. Review.
Cited by
-
Mycobacterium tuberculosis functional genetic diversity, altered drug sensitivity, and precision medicine.Front Cell Infect Microbiol. 2022 Oct 3;12:1007958. doi: 10.3389/fcimb.2022.1007958. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36262182 Free PMC article. Review.
-
Mechanisms of Drug-Induced Tolerance in Mycobacterium tuberculosis.Clin Microbiol Rev. 2020 Oct 14;34(1):e00141-20. doi: 10.1128/CMR.00141-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33055230 Free PMC article. Review.
-
A non-canonical type 2 immune response coordinates tuberculous granuloma formation and epithelialization.Cell. 2021 Apr 1;184(7):1757-1774.e14. doi: 10.1016/j.cell.2021.02.046. Epub 2021 Mar 23. Cell. 2021. PMID: 33761328 Free PMC article.
-
Mathematical model and tool to explore shorter multi-drug therapy options for active pulmonary tuberculosis.PLoS Comput Biol. 2020 Aug 18;16(8):e1008107. doi: 10.1371/journal.pcbi.1008107. eCollection 2020 Aug. PLoS Comput Biol. 2020. PMID: 32810158 Free PMC article.
-
Spatiotemporal perspectives on tuberculosis chemotherapy.Curr Opin Microbiol. 2023 Apr;72:102266. doi: 10.1016/j.mib.2023.102266. Epub 2023 Feb 4. Curr Opin Microbiol. 2023. PMID: 36745965 Free PMC article. Review.
References
-
- WHO. 2016. Global Tuberculosis Report. WHO, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

