Use of Proteins Identified through a Functional Genomic Screen To Develop a Protein Subunit Vaccine That Provides Significant Protection against Virulent Streptococcus suis in Pigs
- PMID: 29203546
- PMCID: PMC5820948
- DOI: 10.1128/IAI.00559-17
Use of Proteins Identified through a Functional Genomic Screen To Develop a Protein Subunit Vaccine That Provides Significant Protection against Virulent Streptococcus suis in Pigs
Abstract
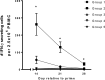
Streptococcus suis is a bacterium that is commonly carried in the respiratory tract and that is also one of the most important invasive pathogens of swine, commonly causing meningitis, arthritis, and septicemia. Due to the existence of many serotypes and a wide range of immune evasion capabilities, efficacious vaccines are not readily available. The selection of S. suis protein candidates for inclusion in a vaccine was accomplished by identifying fitness genes through a functional genomics screen and selecting conserved predicted surface-associated proteins. Five candidate proteins were selected for evaluation in a vaccine trial and administered both intranasally and intramuscularly with one of two different adjuvant formulations. Clinical protection was evaluated by subsequent intranasal challenge with virulent S. suis While subunit vaccination with the S. suis proteins induced IgG antibodies to each individual protein and a cellular immune response to the pool of proteins and provided substantial protection from challenge with virulent S. suis, the immune response elicited and the degree of protection were dependent on the parenteral adjuvant given. Subunit vaccination induced IgG reactive against different S. suis serotypes, indicating a potential for cross protection.
Keywords: Streptococcus suis; adjuvant; functional genomics; subunit; vaccine.
Copyright © 2018 American Society for Microbiology.
Figures





References
-
- Holden MT, Hauser H, Sanders M, Ngo TH, Cherevach I, Cronin A, Goodhead I, Mungall K, Quail MA, Price C, Rabbinowitsch E, Sharp S, Croucher NJ, Chieu TB, Mai NT, Diep TS, Chinh NT, Kehoe M, Leigh JA, Ward PN, Dowson CG, Whatmore AM, Chanter N, Iversen P, Gottschalk M, Slater JD, Smith HE, Spratt BG, Xu J, Ye C, Bentley S, Barrell BG, Schultsz C, Maskell DJ, Parkhill J. 2009. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS One 4:e6072. doi: 10.1371/journal.pone.0006072. - DOI - PMC - PubMed
-
- Ngo TH, Tran TB, Tran TT, Nguyen VD, Campbell J, Pham HA, Huynh HT, Nguyen VV, Bryant JE, Tran TH, Farrar J, Schultsz C. 2011. Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in southern Vietnam. PLoS One 6:e17943. doi: 10.1371/journal.pone.0017943. - DOI - PMC - PubMed
-
- Hill JE, Gottschalk M, Brousseau R, Harel J, Hemmingsen SM, Goh SH. 2005. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet Microbiol 107:63–69. doi: 10.1016/j.vetmic.2005.01.003. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/G018553/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G019177/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G020744/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G019274/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

