Epigenetic therapy activates type I interferon signaling in murine ovarian cancer to reduce immunosuppression and tumor burden
- PMID: 29203668
- PMCID: PMC5754782
- DOI: 10.1073/pnas.1712514114
Epigenetic therapy activates type I interferon signaling in murine ovarian cancer to reduce immunosuppression and tumor burden
Abstract
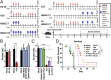
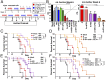
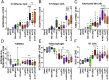
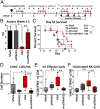
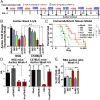
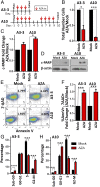
Ovarian cancer is the most lethal of all gynecological cancers, and there is an urgent unmet need to develop new therapies. Epithelial ovarian cancer (EOC) is characterized by an immune suppressive microenvironment, and response of ovarian cancers to immune therapies has thus far been disappointing. We now find, in a mouse model of EOC, that clinically relevant doses of DNA methyltransferase and histone deacetylase inhibitors (DNMTi and HDACi, respectively) reduce the immune suppressive microenvironment through type I IFN signaling and improve response to immune checkpoint therapy. These data indicate that the type I IFN response is required for effective in vivo antitumorigenic actions of the DNMTi 5-azacytidine (AZA). Through type I IFN signaling, AZA increases the numbers of CD45+ immune cells and the percentage of active CD8+ T and natural killer (NK) cells in the tumor microenvironment, while reducing tumor burden and extending survival. AZA also increases viral defense gene expression in both tumor and immune cells, and reduces the percentage of macrophages and myeloid-derived suppressor cells in the tumor microenvironment. The addition of an HDACi to AZA enhances the modulation of the immune microenvironment, specifically increasing T and NK cell activation and reducing macrophages over AZA treatment alone, while further increasing the survival of the mice. Finally, a triple combination of DNMTi/HDACi plus the immune checkpoint inhibitor α-PD-1 provides the best antitumor effect and longest overall survival, and may be an attractive candidate for future clinical trials in ovarian cancer.
Keywords: 5-azacytidine; histone deacetylase inhibitors; immunosuppression; ovarian cancer; type I interferon.
Conflict of interest statement
Conflict of interest statement: S.B.B. and C.A.Z. have a collaborative research agreement with Janssen. V.B., K.R.W., G.S.C., and K.E.B. are employed by Janssen.
Figures

Comment in
-
Reply to Haffner et al.: DNA hypomethylation renders tumors more immunogenic.Proc Natl Acad Sci U S A. 2018 Sep 11;115(37):E8583-E8584. doi: 10.1073/pnas.1811015115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181297 Free PMC article. No abstract available.
-
Hypomethylation, endogenous retrovirus expression, and interferon signaling in testicular germ cell tumors.Proc Natl Acad Sci U S A. 2018 Sep 11;115(37):E8580-E8582. doi: 10.1073/pnas.1803292115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181298 Free PMC article. No abstract available.
Similar articles
-
Epigenetic Therapies in Ovarian Cancer Alter Repetitive Element Expression in a TP53-Dependent Manner.Cancer Res. 2021 Oct 15;81(20):5176-5189. doi: 10.1158/0008-5472.CAN-20-4243. Epub 2021 Aug 25. Cancer Res. 2021. PMID: 34433584 Free PMC article.
-
Combining DNMT and HDAC6 inhibitors increases anti-tumor immune signaling and decreases tumor burden in ovarian cancer.Sci Rep. 2020 Feb 26;10(1):3470. doi: 10.1038/s41598-020-60409-4. Sci Rep. 2020. PMID: 32103105 Free PMC article.
-
Epigenetic modifiers upregulate MHC II and impede ovarian cancer tumor growth.Oncotarget. 2017 Jul 4;8(27):44159-44170. doi: 10.18632/oncotarget.17395. Oncotarget. 2017. PMID: 28498806 Free PMC article.
-
Epi-drugs in combination with immunotherapy: a new avenue to improve anticancer efficacy.Clin Epigenetics. 2017 May 30;9:59. doi: 10.1186/s13148-017-0358-y. eCollection 2017. Clin Epigenetics. 2017. PMID: 28572863 Free PMC article. Review.
-
Strengthening the AntiTumor NK Cell Function for the Treatment of Ovarian Cancer.Int J Mol Sci. 2019 Feb 19;20(4):890. doi: 10.3390/ijms20040890. Int J Mol Sci. 2019. PMID: 30791364 Free PMC article. Review.
Cited by
-
The role of caspase-8 in the tumor microenvironment of ovarian cancer.Cancer Metastasis Rev. 2021 Mar;40(1):303-318. doi: 10.1007/s10555-020-09935-1. Epub 2020 Oct 7. Cancer Metastasis Rev. 2021. PMID: 33026575 Free PMC article. Review.
-
Correlation of LAMA3 with onset and prognosis of ovarian cancer.Oncol Lett. 2019 Sep;18(3):2813-2818. doi: 10.3892/ol.2019.10600. Epub 2019 Jul 10. Oncol Lett. 2019. PMID: 31402958 Free PMC article.
-
Combining epigenetic drugs with other therapies for solid tumours - past lessons and future promise.Nat Rev Clin Oncol. 2020 Feb;17(2):91-107. doi: 10.1038/s41571-019-0267-4. Epub 2019 Sep 30. Nat Rev Clin Oncol. 2020. PMID: 31570827 Review.
-
Comprehensive of N1-Methyladenosine Modifications Patterns and Immunological Characteristics in Ovarian Cancer.Front Immunol. 2021 Oct 29;12:746647. doi: 10.3389/fimmu.2021.746647. eCollection 2021. Front Immunol. 2021. PMID: 34777359 Free PMC article.
-
Methylation across the central dogma in health and diseases: new therapeutic strategies.Signal Transduct Target Ther. 2023 Aug 25;8(1):310. doi: 10.1038/s41392-023-01528-y. Signal Transduct Target Ther. 2023. PMID: 37620312 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

