The heat-shock, or HSF1-mediated proteotoxic stress, response in cancer: from proteomic stability to oncogenesis
- PMID: 29203710
- PMCID: PMC5717525
- DOI: 10.1098/rstb.2016.0525
The heat-shock, or HSF1-mediated proteotoxic stress, response in cancer: from proteomic stability to oncogenesis
Abstract
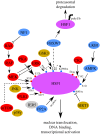
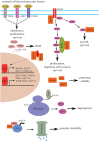
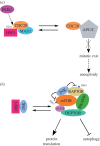
The heat-shock, or HSF1-mediated proteotoxic stress, response (HSR/HPSR) is characterized by induction of heat-shock proteins (HSPs). As molecular chaperones, HSPs facilitate the folding, assembly, transportation and degradation of other proteins. In mammals, heat shock factor 1 (HSF1) is the master regulator of this ancient transcriptional programme. Upon proteotoxic insults, the HSR/HPSR is essential to proteome homeostasis, or proteostasis, thereby resisting stress and antagonizing protein misfolding diseases and ageing. Contrasting with these benefits, an unexpected pro-oncogenic role of the HSR/HPSR is unfolding. Whereas HSF1 remains latent in primary cells without stress, it becomes constitutively activated within malignant cells, rendering them addicted to HSF1 for their growth and survival. Highlighting the HSR/HPSR as an integral component of the oncogenic network, several key pathways governing HSF1 activation by environmental stressors are causally implicated in malignancy. Importantly, HSF1 impacts the cancer proteome systemically. By suppressing tumour-suppressive amyloidogenesis, HSF1 preserves cancer proteostasis to support the malignant state, both providing insight into how HSF1 enables tumorigenesis and suggesting disruption of cancer proteostasis as a therapeutic strategy. This review provides an overview of the role of HSF1 in oncogenesis, mechanisms underlying its constitutive activation within cancer cells and its pro-oncogenic action, as well as potential HSF1-targeting strategies.This article is part of the theme issue 'Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective'.
Keywords: HSF1; amyloids; proteostasis; proteotoxic stress; the heat-shock response; tumorigenesis.
© 2017 The Author(s).
Conflict of interest statement
I declare I have no competing interests.
Figures
Similar articles
-
Heat shock factor 1 (HSF1)-targeted anticancer therapeutics: overview of current preclinical progress.Expert Opin Ther Targets. 2019 May;23(5):369-377. doi: 10.1080/14728222.2019.1602119. Epub 2019 Apr 7. Expert Opin Ther Targets. 2019. PMID: 30931649 Review.
-
HSF1: Guardian of Proteostasis in Cancer.Trends Cell Biol. 2016 Jan;26(1):17-28. doi: 10.1016/j.tcb.2015.10.011. Epub 2015 Nov 18. Trends Cell Biol. 2016. PMID: 26597576 Free PMC article. Review.
-
Rethinking HSF1 in Stress, Development, and Organismal Health.Trends Cell Biol. 2017 Dec;27(12):895-905. doi: 10.1016/j.tcb.2017.08.002. Epub 2017 Sep 7. Trends Cell Biol. 2017. PMID: 28890254 Free PMC article. Review.
-
HSF1: Primary Factor in Molecular Chaperone Expression and a Major Contributor to Cancer Morbidity.Cells. 2020 Apr 22;9(4):1046. doi: 10.3390/cells9041046. Cells. 2020. PMID: 32331382 Free PMC article. Review.
-
An HSF1-JMJD6-HSP feedback circuit promotes cell adaptation to proteotoxic stress.Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2313370121. doi: 10.1073/pnas.2313370121. Epub 2024 Jul 10. Proc Natl Acad Sci U S A. 2024. PMID: 38985769 Free PMC article.
Cited by
-
Glutamine sustains energy metabolism and alleviates liver injury in burn sepsis by promoting the assembly of mitochondrial HSP60-HSP10 complex via SIRT4 dependent protein deacetylation.Redox Rep. 2024 Dec;29(1):2312320. doi: 10.1080/13510002.2024.2312320. Epub 2024 Feb 8. Redox Rep. 2024. PMID: 38329114 Free PMC article.
-
Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy.Cells. 2020 Apr 6;9(4):892. doi: 10.3390/cells9040892. Cells. 2020. PMID: 32268506 Free PMC article. Review.
-
Heat shock factor 1 inhibition enhances the effects of modulated electro hyperthermia in a triple negative breast cancer mouse model.Sci Rep. 2024 Apr 8;14(1):8241. doi: 10.1038/s41598-024-57659-x. Sci Rep. 2024. PMID: 38589452 Free PMC article.
-
HSF1 physically neutralizes amyloid oligomers to empower overgrowth and bestow neuroprotection.Sci Adv. 2020 Nov 11;6(46):eabc6871. doi: 10.1126/sciadv.abc6871. Print 2020 Nov. Sci Adv. 2020. PMID: 33177089 Free PMC article.
-
The stress response regulator HSF1 modulates natural killer cell anti-tumour immunity.Nat Cell Biol. 2024 Oct;26(10):1734-1744. doi: 10.1038/s41556-024-01490-z. Epub 2024 Sep 2. Nat Cell Biol. 2024. PMID: 39223375 Free PMC article.
References
-
- Cannon WB. 1932. The wisdom of the body. New York, NY: WW Norton & Co.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

