Exercise, heat shock proteins and insulin resistance
- PMID: 29203714
- PMCID: PMC5717529
- DOI: 10.1098/rstb.2016.0529
Exercise, heat shock proteins and insulin resistance
Abstract
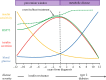
Best known as chaperones, heat shock proteins (HSPs) also have roles in cell signalling and regulation of metabolism. Rodent studies demonstrate that heat treatment, transgenic overexpression and pharmacological induction of HSP72 prevent high-fat diet-induced glucose intolerance and skeletal muscle insulin resistance. Overexpression of skeletal muscle HSP72 in mice has been shown to increase endurance running capacity nearly twofold and increase mitochondrial content by 50%. A positive correlation between HSP72 mRNA expression and mitochondrial enzyme activity has been observed in human skeletal muscle, and HSP72 expression is markedly decreased in skeletal muscle of insulin resistant and type 2 diabetic patients. In addition, decreased levels of HSP72 correlate with insulin resistance and non-alcoholic fatty liver disease progression in livers from obese patients. These data suggest the targeted induction of HSPs could be a therapeutic approach for preventing metabolic disease by maintaining the body's natural stress response. Exercise elicits a number of metabolic adaptations and is a powerful tool in the prevention and treatment of insulin resistance. Exercise training is also a stimulus for increased HSP expression. Although the underlying mechanism(s) for exercise-induced HSP expression are currently unknown, the HSP response may be critical for the beneficial metabolic effects of exercise. Exercise-induced extracellular HSP release may also contribute to metabolic homeostasis by actively restoring HSP72 content in insulin resistant tissues containing low endogenous levels of HSPs.This article is part of the theme issue 'Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective'.
Keywords: aerobic capacity; heat treatment; inflammation; mitochondria; skeletal muscle.
© 2017 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures
References
-
- CDC. 2014. National Diabetes Statistics Report, 2014. Atlanta, GA: Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, US Department of Health & Human Services. https://stacks.cdc.gov/view/cdc/23442/cdc_23442_DS1.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

