The role of heat shock proteins and co-chaperones in heart failure
- PMID: 29203715
- PMCID: PMC5717530
- DOI: 10.1098/rstb.2016.0530
The role of heat shock proteins and co-chaperones in heart failure
Abstract
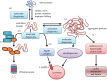
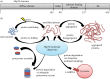
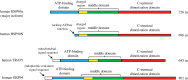
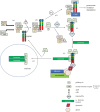
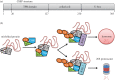
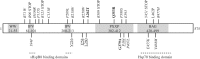
The ongoing contractile and metabolic demands of the heart require a tight control over protein quality control, including the maintenance of protein folding, turnover and synthesis. In heart disease, increases in mechanical and oxidative stresses, post-translational modifications (e.g., phosphorylation), for example, decrease protein stability to favour misfolding in myocardial infarction, heart failure or ageing. These misfolded proteins are toxic to cardiomyocytes, directly contributing to the common accumulation found in human heart failure. One of the critical class of proteins involved in protecting the heart against these threats are molecular chaperones, including the heat shock protein70 (HSP70), HSP90 and co-chaperones CHIP (carboxy terminus of Hsp70-interacting protein, encoded by the Stub1 gene) and BAG-3 (BCL2-associated athanogene 3). Here, we review their emerging roles in the maintenance of cardiomyocytes in human and experimental models of heart failure, including their roles in facilitating the removal of misfolded and degraded proteins, inhibiting apoptosis and maintaining the structural integrity of the sarcomere and regulation of nuclear receptors. Furthermore, we discuss emerging evidence of increased expression of extracellular HSP70, HSP90 and BAG-3 in heart failure, with complementary independent roles from intracellular functions with important therapeutic and diagnostic considerations. While our understanding of these major HSPs in heart failure is incomplete, there is a clear potential role for therapeutic modulation of HSPs in heart failure with important contextual considerations to counteract the imbalance of protein damage and endogenous protein quality control systems.This article is part of the theme issue 'Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective'.
Keywords: BAG-3; HSP70; HSP90; Stub1; carboxy terminus of HSP70-interacting protein; heart failure.
© 2017 The Author(s).
Conflict of interest statement
The authors do not have competing interests to report.
Figures

Similar articles
-
Heat shock proteins and their expression in primary murine cardiac cell populations during ischemia and reperfusion.Mol Cell Biochem. 2020 Jan;464(1-2):21-26. doi: 10.1007/s11010-019-03645-1. Epub 2019 Nov 1. Mol Cell Biochem. 2020. PMID: 31677029 Review.
-
The role of heat shock proteins in the pathogenesis of heart failure (Review).Int J Mol Med. 2023 Nov;52(5):106. doi: 10.3892/ijmm.2023.5309. Epub 2023 Sep 29. Int J Mol Med. 2023. PMID: 37772383 Free PMC article. Review.
-
Heat shock proteins and cancer: intracellular chaperones or extracellular signalling ligands?Philos Trans R Soc Lond B Biol Sci. 2018 Jan 19;373(1738):20160524. doi: 10.1098/rstb.2016.0524. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29203709 Free PMC article. Review.
-
Roles of molecular chaperones in the nervous system.Brain Res Bull. 2000 Sep 15;53(2):141-6. doi: 10.1016/s0361-9230(00)00325-7. Brain Res Bull. 2000. PMID: 11044589 Review.
-
General Structural and Functional Features of Molecular Chaperones.Adv Exp Med Biol. 2021;1340:11-73. doi: 10.1007/978-3-030-78397-6_2. Adv Exp Med Biol. 2021. PMID: 34569020
Cited by
-
Genomic and Non-Genomic Regulatory Mechanisms of the Cardiac Sodium Channel in Cardiac Arrhythmias.Int J Mol Sci. 2022 Jan 26;23(3):1381. doi: 10.3390/ijms23031381. Int J Mol Sci. 2022. PMID: 35163304 Free PMC article. Review.
-
Targeting Extracellular Heat Shock Protein 70 Ameliorates Doxorubicin-Induced Heart Failure Through Resolution of Toll-Like Receptor 2-Mediated Myocardial Inflammation.J Am Heart Assoc. 2019 Oct 15;8(20):e012338. doi: 10.1161/JAHA.119.012338. Epub 2019 Oct 2. J Am Heart Assoc. 2019. PMID: 31576776 Free PMC article.
-
Acquired Resilience: An Evolved System of Tissue Protection in Mammals.Dose Response. 2018 Dec 27;16(4):1559325818803428. doi: 10.1177/1559325818803428. eCollection 2018 Oct-Dec. Dose Response. 2018. PMID: 30627064 Free PMC article. Review.
-
Modeling of LMNA-Related Dilated Cardiomyopathy Using Human Induced Pluripotent Stem Cells.Cells. 2019 Jun 15;8(6):594. doi: 10.3390/cells8060594. Cells. 2019. PMID: 31208058 Free PMC article.
-
Asb10 accelerates pathological cardiac remodeling by stabilizing HSP70.Cell Death Dis. 2025 May 22;16(1):409. doi: 10.1038/s41419-025-07735-5. Cell Death Dis. 2025. PMID: 40399264 Free PMC article.
References
-
- WHO. 2017. Cardiovascular diseases (CVDs) Fact Sheet. See http://www.who.int/mediacentre/factsheets/fs317/en/. (accessed 8 July 2017).
-
- Ni H, Xu J. 2015. Recent trends in heart failure-related mortality: United States, 2000–2014. NCHS Data Brief. 231, 1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

