Age-dependent increase of oxidative stress regulates microRNA-29 family preserving cardiac health
- PMID: 29203887
- PMCID: PMC5715159
- DOI: 10.1038/s41598-017-16829-w
Age-dependent increase of oxidative stress regulates microRNA-29 family preserving cardiac health
Abstract
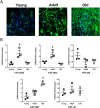
The short-lived turquoise killifish Nothobranchius furzeri (Nfu) is a valid model for aging studies. Here, we investigated its age-associated cardiac function. We observed oxidative stress accumulation and an engagement of microRNAs (miRNAs) in the aging heart. MiRNA-sequencing of 5 week (young), 12-21 week (adult) and 28-40 week (old) Nfu hearts revealed 23 up-regulated and 18 down-regulated miRNAs with age. MiR-29 family turned out as one of the most up-regulated miRNAs during aging. MiR-29 family increase induces a decrease of known targets like collagens and DNA methyl transferases (DNMTs) paralleled by 5´methyl-cytosine (5mC) level decrease. To further investigate miR-29 family role in the fish heart we generated a transgenic zebrafish model where miR-29 was knocked-down. In this model we found significant morphological and functional cardiac alterations and an impairment of oxygen dependent pathways by transcriptome analysis leading to hypoxic marker up-regulation. To get insights the possible hypoxic regulation of miR-29 family, we exposed human cardiac fibroblasts to 1% O2 levels. In hypoxic condition we found miR-29 down-modulation responsible for the accumulation of collagens and 5mC. Overall, our data suggest that miR-29 family up-regulation might represent an endogenous mechanism aimed at ameliorating the age-dependent cardiac damage leading to hypertrophy and fibrosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


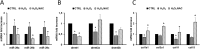




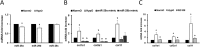
Similar articles
-
MicroRNA-223 Regulates Cardiac Fibrosis After Myocardial Infarction by Targeting RASA1.Cell Physiol Biochem. 2018;46(4):1439-1454. doi: 10.1159/000489185. Epub 2018 Apr 19. Cell Physiol Biochem. 2018. PMID: 29689569
-
MicroRNA-323a-3p Promotes Pressure Overload-Induced Cardiac Fibrosis by Targeting TIMP3.Cell Physiol Biochem. 2018;50(6):2176-2187. doi: 10.1159/000495059. Epub 2018 Nov 9. Cell Physiol Biochem. 2018. PMID: 30415251
-
Age-dependent regulation of tumor-related microRNAs in the brain of the annual fish Nothobranchius furzeri.Mech Ageing Dev. 2012 May;133(5):226-33. doi: 10.1016/j.mad.2012.03.015. Epub 2012 Apr 6. Mech Ageing Dev. 2012. PMID: 22487494
-
Na/K-ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts.Physiol Genomics. 2016 Mar;48(3):220-9. doi: 10.1152/physiolgenomics.00116.2015. Epub 2015 Dec 23. Physiol Genomics. 2016. PMID: 26702050 Free PMC article.
-
MiR-19b-3p Regulates MAPK1 Expression in Embryonic Fibroblasts from the Great Tit (Parus Major) Under Hypoxic Conditions.Cell Physiol Biochem. 2018;46(2):546-560. doi: 10.1159/000488621. Epub 2018 Mar 28. Cell Physiol Biochem. 2018. PMID: 29617681
Cited by
-
Alternative Animal Models of Aging Research.Front Mol Biosci. 2021 May 17;8:660959. doi: 10.3389/fmolb.2021.660959. eCollection 2021. Front Mol Biosci. 2021. PMID: 34079817 Free PMC article. Review.
-
Deficiency of miR-29a/b1 leads to premature aging and dopaminergic neuroprotection in mice.Front Mol Neurosci. 2022 Oct 6;15:978191. doi: 10.3389/fnmol.2022.978191. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36277485 Free PMC article.
-
Differences in microRNA-29 and Pro-fibrotic Gene Expression in Mouse and Human Hypertrophic Cardiomyopathy.Front Cardiovasc Med. 2019 Dec 17;6:170. doi: 10.3389/fcvm.2019.00170. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 31921893 Free PMC article.
-
Bisphenol A, Tobacco Smoke, and Age as Predictors of Oxidative Stress in Children and Adolescents.Int J Environ Res Public Health. 2019 Jun 6;16(11):2025. doi: 10.3390/ijerph16112025. Int J Environ Res Public Health. 2019. PMID: 31174388 Free PMC article.
-
Roles of Biomarkers in Myocardial Fibrosis.Aging Dis. 2020 Oct 1;11(5):1157-1174. doi: 10.14336/AD.2020.0604. eCollection 2020 Oct. Aging Dis. 2020. PMID: 33014530 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

