Combining intratumoral Treg depletion with androgen deprivation therapy (ADT): preclinical activity in the Myc-CaP model
- PMID: 29203894
- PMCID: PMC5897134
- DOI: 10.1038/s41391-017-0013-x
Combining intratumoral Treg depletion with androgen deprivation therapy (ADT): preclinical activity in the Myc-CaP model
Abstract
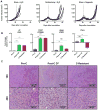
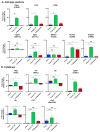
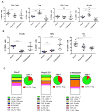
Background: Immune checkpoint blockade has shown promising antitumor activity against a variety of tumor types. However, responses in castration-resistant prostate cancer remain relatively rare-potentially due to low baseline levels of infiltration. Using an immunocompetent cMyc-driven model (Myc-CaP), we sought to understand the immune infiltrate induced by androgen deprivation therapy (ADT) and to leverage that infiltration toward therapeutic benefit.
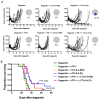
Methods: Using flow cytometry, qPCR and IHC, we quantified ADT-induced immune infiltration in terms of cell type and function. Preclinical treatment studies tested the combinatorial effects of ADT and immune checkpoint blockade using tumor outgrowth and overall survival as end points.
Results: ADT induces a complex pro-inflammatory infiltrate. This pro-inflammatory infiltrate was apparent in the early postcastration period but diminished as castration resistance emerged. Combining ADT with tumor-infiltrating regulatory T cell (Treg) depletion using a depleting anti-CTLA-4 antibody significantly delayed the development of castration resistance and prolonged survival of a fraction of tumor-bearing mice. Immunotherapy as a monotherapy failed to provide a survival benefit and was ineffective if not administered in the peri-castration period.
Conclusions: The immune infiltrate induced by ADT is diverse and varies over time. Therapeutic strategies focusing on depleting Tregs in the peri-castration period are of particular interest.
Conflict of interest statement
Conflicts of Interest: CGD has served as a paid consultant to Agenus, Bristol Myers Squibb, Compugen, Dendreon, Merck and Roche Genentech, and has received sponsored research funding (institutional) from Bristol Myers Squibb under the International Immuno-Oncology Network (IIoN).
Figures




References
-
- Callahan MK, Postow MA, Wolchok JD. Targeting T Cell Co-receptors for Cancer Therapy. Immunity. 2016;44(5):1069–78. - PubMed
-
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–12. - PMC - PubMed
-
- Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol. 2017;35(1):40–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

