LETM1-Mediated K+ and Na+ Homeostasis Regulates Mitochondrial Ca2+ Efflux
- PMID: 29204122
- PMCID: PMC5698270
- DOI: 10.3389/fphys.2017.00839
LETM1-Mediated K+ and Na+ Homeostasis Regulates Mitochondrial Ca2+ Efflux
Abstract
Ca2+ transport across the inner membrane of mitochondria (IMM) is of major importance for their functions in bioenergetics, cell death and signaling. It is therefore tightly regulated. It has been recently proposed that LETM1—an IMM protein with a crucial role in mitochondrial K+/H+ exchange and volume homeostasis—also acts as a Ca2+/H+ exchanger. Here we show for the first time that lowering LETM1 gene expression by shRNA hampers mitochondrial K+/H+ and Na+/H+ exchange. Decreased exchange activity resulted in matrix K+ accumulation in these mitochondria. Furthermore, LETM1 depletion selectively decreased Na+/Ca2+ exchange mediated by NCLX, as observed in the presence of ruthenium red, a blocker of the Mitochondrial Ca2+ Uniporter (MCU). These data confirm a key role of LETM1 in monovalent cation homeostasis, and suggest that the effects of its modulation on mitochondrial transmembrane Ca2+ fluxes may reflect those on Na+/H+ exchange activity.
Keywords: LETM1; calcium; mitochondrial cation/proton exchange; mitochondrial volume homeostasis; potassium; sodium.
Figures
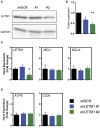


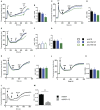
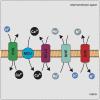
References
-
- Bernardi P. (1999). Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 79, 1127–1155. - PubMed
-
- Blomeyer C. A., Bazil J. N., Stowe D. F., Dash R. K., Camara A. K. (2016). Mg(2+) differentially regulates two modes of mitochondrial Ca(2+) uptake in isolated cardiac mitochondria: implications for mitochondrial Ca(2+) sequestration. J. Bioenerg. Biomembr. 48, 175–188. 10.1007/s10863-016-9644-1 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

