Mitochondria: A Common Target for Genetic Mutations and Environmental Toxicants in Parkinson's Disease
- PMID: 29204154
- PMCID: PMC5698285
- DOI: 10.3389/fgene.2017.00177
Mitochondria: A Common Target for Genetic Mutations and Environmental Toxicants in Parkinson's Disease
Abstract
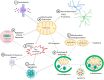
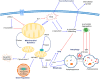
Parkinson's disease (PD) is a devastating neurological movement disorder. Since its first discovery 200 years ago, genetic and environmental factors have been identified to play a role in PD development and progression. Although genetic studies have been the predominant driving force in PD research over the last few decades, currently only a small fraction of PD cases can be directly linked to monogenic mutations. The remaining cases have been attributed to other risk associated genes, environmental exposures and gene-environment interactions, making PD a multifactorial disorder with a complex etiology. However, enormous efforts from global research have yielded significant insights into pathogenic mechanisms and potential therapeutic targets for PD. This review will highlight mitochondrial dysfunction as a common pathway involved in both genetic mutations and environmental toxicants linked to PD.
Keywords: Parkinson’s disease; environmental toxins; gene–environment interaction; mitochondrial dynamics; mitochondrial dysfunction; neurodegeneration; neurotoxicity.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

