Cytotoxic effects of the newly-developed chemotherapeutic agents 17-AAG in combination with oxaliplatin and capecitabine in colorectal cancer cell lines
- PMID: 29204180
- PMCID: PMC5691578
- DOI: 10.4103/1735-5362.217432
Cytotoxic effects of the newly-developed chemotherapeutic agents 17-AAG in combination with oxaliplatin and capecitabine in colorectal cancer cell lines
Abstract
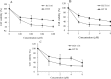
The use of heat shock protein 90 inhibitors like 17-allylamino-17-demethoxy-geldanamycin (17-AAG) has been recently introduced as an attractive anticancer therapy. It has been shown that 17-AAG may potentiate the inhibitory effects of some classical anticolorectal cancer (CRC) agents. In this study, two panels of colorectal carcinoma cell lines were used to evaluate the effects of 17-AAG in combination with capecitabine and oxaliplatin as double and triple combination therapies on the proliferation of CRC cell lines. HT-29 and all HCT-116 cell lines were seeded in culture media in the presence of different doses of the mentioned drugs in single, double, and triple combinations. Water-soluble tetrazolium-1 (WST-1) assay was used to investigate cell proliferation 24 h after treatments. Then, dose-response curves were plotted using WST-1outputs, and IC50 values were determined. For double and triple combinations respectively 0.5 × IC50 and 0.25 × IC50 were used. Data was analyzed with the software CompuSyn. Drug interactions were analyzed using Chou-Talalay method to calculate the combination index (CI).The data revealed that 17-AAG shows a potent synergistic interaction (CI < 1) with oxaliplatin and capecitabine in double combinations (0.5 × IC50) in both cell lines. In the case of triple combinations, the findings showed an antagonistic interaction (CI > 1) in HT-29 and a synergistic effect (CI < 1) in HCT-116 (0.25 × IC50) cell lines. It was concluded that double combinations of 17-AAG with oxaliplatin or capecitabine might be effective against HCT-116 and HT-29 cell lines. However, in triple combinations, positive results were seen only against HCT-116. Further investigation is suggested to confirm the effectiveness of these combinations in clinical trials.
Keywords: 17-AAG; Capecitabine; Colorectal cancer; HCT-116; HT-29; Oxaliplatin.
Figures
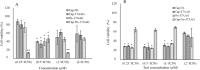
Similar articles
-
Dihydropyrimidine Dehydrogenase Levels in Colorectal Cancer Cells Treated with a Combination of Heat Shock Protein 90 Inhibitor and Oxaliplatin or Capecitabine.Adv Pharm Bull. 2019 Aug;9(3):439-444. doi: 10.15171/apb.2019.052. Epub 2019 Aug 1. Adv Pharm Bull. 2019. PMID: 31592113 Free PMC article.
-
Anti-cancer effects of chemotherapeutic agent; 17-AAG, in combined with gold nanoparticles and irradiation in human colorectal cancer cells.Daru. 2019 Jun;27(1):111-119. doi: 10.1007/s40199-019-00251-w. Epub 2019 Mar 5. Daru. 2019. PMID: 30835081 Free PMC article.
-
A molecular basis for the synergy between 17‑allylamino‑17‑demethoxy geldanamycin with Capecitabine and Irinotecan in human colorectal cancer cells through VEFG and MMP-9 gene expression.Gene. 2019 Feb 5;684:30-38. doi: 10.1016/j.gene.2018.10.016. Epub 2018 Oct 11. Gene. 2019. PMID: 30315927
-
New oxaliplatin-based combinations in the treatment of colorectal cancer.Colorectal Dis. 2003 Nov;5 Suppl 3:1-9. doi: 10.1046/j.1463-1318.5.s3.2.x. Colorectal Dis. 2003. PMID: 23573555 Review.
-
Capecitabine plus oxaliplatin for the treatment of colorectal cancer.Expert Rev Anticancer Ther. 2008 Feb;8(2):161-74. doi: 10.1586/14737140.8.2.161. Expert Rev Anticancer Ther. 2008. PMID: 18279056 Review.
Cited by
-
Fisetin and/or capecitabine causes changes in apoptosis pathways in capecitabine-resistant colorectal cancer cell lines.Naunyn Schmiedebergs Arch Pharmacol. 2024 Oct;397(10):7913-7926. doi: 10.1007/s00210-024-03145-0. Epub 2024 May 15. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38748229 Free PMC article.
-
Dietary silymarin supplementation enhances chemotherapy efficacy of capecitabine and irinotecan and mitigates hepatotoxicity in a mouse model of colon cancer.Res Pharm Sci. 2025 Feb 20;20(1):77-94. doi: 10.4103/RPS.RPS_204_24. eCollection 2025 Feb. Res Pharm Sci. 2025. PMID: 40190825 Free PMC article.
-
Hsp90 Inhibitor; NVP-AUY922 in Combination with Doxorubicin Induces Apoptosis and Downregulates VEGF in MCF-7 Breast Cancer Cell Line.Asian Pac J Cancer Prev. 2020 Jun 1;21(6):1773-1778. doi: 10.31557/APJCP.2020.21.6.1773. Asian Pac J Cancer Prev. 2020. PMID: 32592377 Free PMC article.
-
Dihydropyrimidine Dehydrogenase Levels in Colorectal Cancer Cells Treated with a Combination of Heat Shock Protein 90 Inhibitor and Oxaliplatin or Capecitabine.Adv Pharm Bull. 2019 Aug;9(3):439-444. doi: 10.15171/apb.2019.052. Epub 2019 Aug 1. Adv Pharm Bull. 2019. PMID: 31592113 Free PMC article.
-
The Potential Preventive and Therapeutic Roles of NSAIDs in Prostate Cancer.Cancers (Basel). 2023 Nov 16;15(22):5435. doi: 10.3390/cancers15225435. Cancers (Basel). 2023. PMID: 38001694 Free PMC article. Review.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden. Globocan 2000. Int J Cancer. 2001;94(2):153–156. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. - PubMed
-
- Ulukaya E. Drug response assay: an increasing trend in designation of trailored-chemotherapy for more rational management of cancer patients. Adv Mol Med. 2006;2(2):53–58.
-
- Cao Y, Liao C, Tan A, Liu L, Mo Z, Gao F. Capecitabine plus oxaliplatin vs. fluorouracil plus oxaliplatin as first line treatment for metastatic colorectal cancer meta-analysis of six randomized trials. Colorectal Dis. 2010;12(1):16–23. - PubMed
-
- Zhao G, Gao P, Yang KH, Tian JH, Ma B. Capecitabine/oxaliplatin as first-line treatment for metastatic colorectal cancer: a meta-analysis. Colorectal Dis. 2010;12(7):615–623. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

