Paracoccidioidomycosis: Current Perspectives from Brazil
- PMID: 29204222
- PMCID: PMC5695158
- DOI: 10.2174/1874285801711010224
Paracoccidioidomycosis: Current Perspectives from Brazil
Abstract
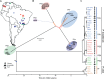
Background: This review article summarizes and updates the knowledge on paracoccidioidomycosis. P lutzii and the cryptic species of P. brasiliensis and their geographical distribution in Latin America, explaining the difficulties observed in the serological diagnosis.
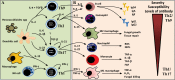
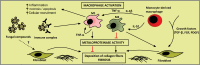
Objectives: Emphasis has been placed on some genetic factors as predisposing condition for paracoccidioidomycosis. Veterinary aspects were focused, showing the wide distribution of infection among animals. The cell-mediated immunity was better characterized, incorporating the recent findings.
Methods: Serological methods for diagnosis were also compared for their parameters of accuracy, including the analysis of relapse.
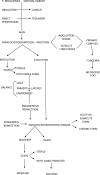
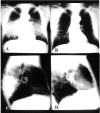
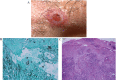
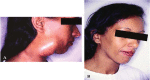
Results: Clinical forms have been better classified in order to include the pictures less frequently observesiod.
Conclusion: Itraconazole and the trimethoprim-sulfamethoxazole combination was compared regarding efficacy, effectiveness and safety, demonstrating that azole should be the first choice in the treatment of paracoccidioidomycosis.
Keywords: Antifungal compounds; Endemic mycosis; Paracoccidioides brasiliensis; Paracoccidioides lutzii; Paracoccidioidmycosis; Systemic mycosis.
Figures



References
-
- Lutz A. Uma mycose pseudococcidica localisada na bocca e observada no Brazil. Contribuição ao conhecimento das hiphoblastomycoses americanas. Brazil-méd. 1908;22(121-124):141–144.
-
- Splendore A. Zymonematosi com localizzazione nella cavità della bocca, osservata in Brasile. Bull. Soc. Pathol. Exot. 1912;5:313–319.
-
- Almeida FP. Estudos comparativos de granuloma coccidióidico nos Estados Unidos e no Brasil: novo gênero para o parasito brasileiro. An Fac Med S Paulo. 1930;5:125–141.
-
- Jordan E.P. Standard nomenclature of diseases and standard nomenclature of operations. Chicago: American Medical Association; 1942.
-
- Paracoccidioidomycosis. Proceedings of the first pan american symposium, medellín (Colombia). PAHO Sci Publ. 1971;254
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
