Laser-emission imaging of nuclear biomarkers for high-contrast cancer screening and immunodiagnosis
- PMID: 29204310
- PMCID: PMC5711465
- DOI: 10.1038/s41551-017-0128-3
Laser-emission imaging of nuclear biomarkers for high-contrast cancer screening and immunodiagnosis
Abstract
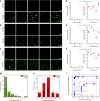
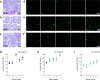
Detection of nuclear biomarkers such as nucleic acids and nuclear proteins is critical for early-stage cancer diagnosis and prognosis. Conventional methods relying on morphological assessment of cell nuclei in histopathology slides may be subjective, whereas colorimetric immunohistochemical and fluorescence-based imaging are limited by strong light absorption, broad-emission bands and low contrast. Here, we describe the development and use of a scanning laser-emission-based microscope that maps lasing emissions from nuclear biomarkers in human tissues. 41 tissue samples from 35 patients labelled with site-specific and biomarker-specific antibody-conjugated dyes were sandwiched in a Fabry-Pérot microcavity while an excitation laser beam built a laser-emission image. We observed multiple sub-cellular lasing emissions from cancer cell nuclei, with a threshold of tens of μJ/mm2, sub-micron resolution (<700 nm), and a lasing band in the few-nanometre range. Different lasing thresholds of nuclei in cancer and normal tissues enabled the identification and multiplexed detection of nuclear proteomic biomarkers, with a high sensitivity for early-stage cancer diagnosis. Laser-emission-based cancer screening and immunodiagnosis might find use in precision medicine and facilitate research in cell biology.
Keywords: Fabry-Pérot cavity; cancer; diagnosis; lasers; microscopy; multiplexed detection; nuclear biomarkers; nucleic acids; sub-cellular resolution; tissues.
Conflict of interest statement
Additional Information. The authors declare no competing financial interests.
Figures






References
-
- Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–687. - PubMed
-
- Swarup V, Rajeswari M. Circulating (cell-free) nucleic acids–a promising, non-invasive tool for early detection of several human diseases. FEBS Lett. 2007;581:795–799. - PubMed
-
- Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

