Self-renewing Monolayer of Primary Colonic or Rectal Epithelial Cells
- PMID: 29204504
- PMCID: PMC5710741
- DOI: 10.1016/j.jcmgh.2017.02.011
Self-renewing Monolayer of Primary Colonic or Rectal Epithelial Cells
Abstract
Background & aims: Three-dimensional organoid culture has fundamentally changed the in vitro study of intestinal biology enabling novel assays; however, its use is limited because of an inaccessible luminal compartment and challenges to data gathering in a three-dimensional hydrogel matrix. Long-lived, self-renewing 2-dimensional (2-D) tissue cultured from primary colon cells has not been accomplished.
Methods: The surface matrix and chemical factors that sustain 2-D mouse colonic and human rectal epithelial cell monolayers with cell repertoires comparable to that in vivo were identified.
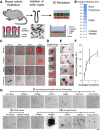
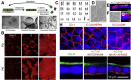
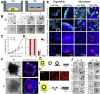
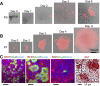
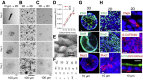
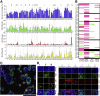
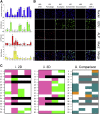
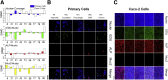
Results: The monolayers formed organoids or colonoids when placed in standard Matrigel culture. As with the colonoids, the monolayers exhibited compartmentalization of proliferative and differentiated cells, with proliferative cells located near the peripheral edges of growing monolayers and differentiated cells predominated in the central regions. Screening of 77 dietary compounds and metabolites revealed altered proliferation or differentiation of the murine colonic epithelium. When exposed to a subset of the compound library, murine organoids exhibited similar responses to that of the monolayer but with differences that were likely attributable to the inaccessible organoid lumen. The response of the human primary epithelium to a compound subset was distinct from that of both the murine primary epithelium and human tumor cells.
Conclusions: This study demonstrates that a self-renewing 2-D murine and human monolayer derived from primary cells can serve as a physiologically relevant assay system for study of stem cell renewal and differentiation and for compound screening. The platform holds transformative potential for personalized and precision medicine and can be applied to emerging areas of disease modeling and microbiome studies.
Keywords: 2-D, two-dimensional; 3-D, three-dimensional; ALP, alkaline phosphatase; CAG, cytomegalovirus enhancer plus chicken actin promoter; CI, confidence interval; Colonic Epithelial Cells; Compound Screening; ECM, extracellular matrix; EDU, 5-ethynyl-2′-deoxyuridine; EGF, epidermal growth factor; ENR-W, cell medium with [Wnt-3A] of 30 ng/mL; ENR-w, cell medium with [Wnt-3A] of 10 ng/mL; HISC, human intestinal stem cell medium; IACUC, Institutional Animal Care and Use Committee; ISC, intestinal stem cell; Monolayer; Organoids; PBS, phosphate-buffered saline; PDMS, polydimethylsiloxane; RFP, red fluorescent protein; SEM, scanning electron microscope; SSMD, strictly standardized mean difference; UNC, University of North Carolina; α-ChgA, anti-chromogranin A; α-Muc2, anti-mucin2.
Figures

References
-
- Grossmann J., Walther K., Artinger M. Progress on isolation and short-term ex-vivo culture of highly purified non-apoptotic human intestinal epithelial cells (IEC) Eur J Cell Biol. 2003;82:262–270. - PubMed
-
- Engle M.J., Goetz G.S., Alpers D.H. Caco-2 cells express a combination of colonocyte and enterocyte phenotypes. J Cell Physiol. 1998;174:362–369. - PubMed
-
- Sun H., Chow E.C.Y., Liu S. The Caco-2 cell monolayer: usefulness and limitations. Expert Opin Drug Metab Toxicol. 2008;4:395–411. - PubMed
-
- Jung P., Sato T., Merlos-Suarez A. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–1227. - PubMed
Supplementary References
-
- Ooi C.C., Good N.M., Williams D.B. Structure-activity relationship of butyrate analogues on apoptosis, proliferation and histone deacetylase activity in HCT-116 human colorectal cancer cells. Clin Exp Pharmacol Physiol. 2010;37:905–911. - PubMed
-
- Malago J.J., Koninkx J., Douma P.M. Differential modulation of enterocyte-like Caco-2 cells after exposure to short-chain fatty acids. Food Additives and Contaminants. 2003;20:427–437. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

