Combination of mAb-AR20.5, anti-PD-L1 and PolyICLC inhibits tumor progression and prolongs survival of MUC1.Tg mice challenged with pancreatic tumors
- PMID: 29204701
- PMCID: PMC7108804
- DOI: 10.1007/s00262-017-2095-7
Combination of mAb-AR20.5, anti-PD-L1 and PolyICLC inhibits tumor progression and prolongs survival of MUC1.Tg mice challenged with pancreatic tumors
Abstract
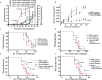
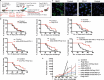
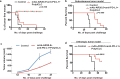
A substantial body of evidence suggests the existence of MUC1-specific antibodies and cytotoxic T cell activities in pancreatic cancer patients. However, tumor-induced immunosuppression renders these responses ineffective. The current study explores a novel therapeutic combination wherein tumor-bearing hosts can be immunologically primed with their own antigen, through opsonization with a tumor antigen-targeted antibody, mAb-AR20.5. We evaluated the efficacy of immunization with this antibody in combination with PolyICLC and anti-PD-L1. The therapeutic combination of mAb-AR20.5 + anti-PD-L1 + PolyICLC induced rejection of human MUC1 expressing tumors and provided a long-lasting, MUC1-specific cellular immune response, which could be adoptively transferred and shown to provide protection against tumor challenge in human MUC1 transgenic (MUC.Tg) mice. Furthermore, antibody depletion studies revealed that CD8 cells were effectors for the MUC1-specific immune response generated by the mAb-AR20.5 + anti-PD-L1 + PolyICLC combination. Multichromatic flow cytometry data analysis demonstrated a significant increase over time in circulating, activated CD8 T cells, CD3+CD4-CD8-(DN) T cells, and mature dendritic cells in mAb-AR20.5 + anti-PD-L1 + PolyICLC combination-treated, tumor-bearing mice, as compared to saline-treated control counterparts. Our study provides a proof of principle that an effective and long-lasting anti-tumor cellular immunity can be achieved in pancreatic tumor-bearing hosts against their own antigen (MUC1), which can be further potentiated using a vaccine adjuvant and an immune checkpoint inhibitor.
Keywords: Anti-PD-L1; CD8 T cells; MUC1; Pancreatic cancer; PolyICLC; mAb-AR20.5 antibody.
Conflict of interest statement
The authors have no conflict of interest.
Figures



Similar articles
-
Anti-PD-L1 prolongs survival and triggers T cell but not humoral anti-tumor immune responses in a human MUC1-expressing preclinical ovarian cancer model.Cancer Immunol Immunother. 2015 Sep;64(9):1095-108. doi: 10.1007/s00262-015-1712-6. Epub 2015 May 22. Cancer Immunol Immunother. 2015. PMID: 25998800 Free PMC article.
-
Influence of organ site and tumor cell type on MUC1-specific tumor immunity.Int Immunol. 2001 Feb;13(2):233-40. doi: 10.1093/intimm/13.2.233. Int Immunol. 2001. PMID: 11157856
-
Characterization of an anti-MUC1 monoclonal antibody with potential as a cancer vaccine.Hybrid Hybridomics. 2001;20(5-6):313-24. doi: 10.1089/15368590152740716. Hybrid Hybridomics. 2001. PMID: 11839249
-
Latest developments in MUC1 immunotherapy.Biochem Soc Trans. 2018 Jun 19;46(3):659-668. doi: 10.1042/BST20170400. Epub 2018 May 21. Biochem Soc Trans. 2018. PMID: 29784646 Free PMC article. Review.
-
111In/125/131I-Labeled anti-mucin-1 murine, chimeric or humanized antibody hPAM4.2011 Jun 16 [updated 2011 Jul 26]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Jun 16 [updated 2011 Jul 26]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21834178 Free Books & Documents. Review.
Cited by
-
89Zr-Labeled AR20.5: A MUC1-Targeting ImmunoPET Probe.Molecules. 2020 May 15;25(10):2315. doi: 10.3390/molecules25102315. Molecules. 2020. PMID: 32429033 Free PMC article.
-
Combination systemic therapies with immune checkpoint inhibitors in pancreatic cancer: overcoming resistance to single-agent checkpoint blockade.Clin Transl Med. 2018 Oct 8;7(1):32. doi: 10.1186/s40169-018-0210-9. Clin Transl Med. 2018. PMID: 30294755 Free PMC article. Review.
-
Macrophages potentiate STAT3 signaling in skeletal muscles and regulate pancreatic cancer cachexia.Cancer Lett. 2020 Aug 1;484:29-39. doi: 10.1016/j.canlet.2020.04.017. Epub 2020 Apr 25. Cancer Lett. 2020. PMID: 32344015 Free PMC article.
-
New avenues for the treatment of immunotherapy-resistant pancreatic cancer.World J Gastrointest Oncol. 2024 Apr 15;16(4):1134-1153. doi: 10.4251/wjgo.v16.i4.1134. World J Gastrointest Oncol. 2024. PMID: 38660642 Free PMC article. Review.
-
Pancreatic Cancer and Its Microenvironment-Recent Advances and Current Controversies.Int J Mol Sci. 2020 May 1;21(9):3218. doi: 10.3390/ijms21093218. Int J Mol Sci. 2020. PMID: 32370075 Free PMC article. Review.
References
-
- Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54:2856–2860. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

