Presynaptic Ethanol Actions: Potential Roles in Ethanol Seeking
- PMID: 29204712
- PMCID: PMC11103585
- DOI: 10.1007/164_2017_76
Presynaptic Ethanol Actions: Potential Roles in Ethanol Seeking
Erratum in
-
Correction to: Presynaptic Ethanol Actions: Potential Roles in Ethanol Seeking.Handb Exp Pharmacol. 2018;248:617. doi: 10.1007/164_2018_189. Handb Exp Pharmacol. 2018. PMID: 30810859
Abstract
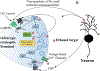
Ethanol produces intoxication through actions on numerous molecular and cellular targets. Adaptations involving these and other targets contribute to chronic drug actions that underlie continued and problematic drinking. Among the mechanisms involved in these ethanol actions are alterations in presynaptic mechanisms of synaptic transmission, including presynaptic protein function and excitation-secretion coupling. At synapses in the central nervous system (CNS), excitation-secretion coupling involves ion channel activation followed by vesicle fusion and neurotransmitter release. These mechanisms are altered by presynaptic neurotransmitter receptors and prominently by G protein-coupled receptors (GPCRs). Studies over the last 20-25 years have revealed that acute ethanol exposure alters neurotransmitter secretion, with especially robust effects on synapses that use the neurotransmitter gamma-aminobutyric acid (GABA). Intracellular signaling pathways involving second messengers such as cyclic AMP and calcium are implicated in these acute ethanol actions. Ethanol-induced release of neuropeptides and small molecule neurotransmitters that act on presynaptic GPCRs also contribute to presynaptic potentiation at synapses in the amygdala and hippocampus and inhibition of GABA release in the striatum. Prolonged exposure to ethanol alters neurotransmitter release at many CNS GABAergic and glutamatergic synapses, and changes in GPCR function are implicated in many of these neuroadaptations. These presynaptic neuroadaptations appear to involve compensation for acute drug effects at some synapses, but "allostatic" effects that result in long-term resetting of synaptic efficacy occur at others. Current investigations are determining how presynaptic neuroadaptations contribute to behavioral changes at different stages of alcohol drinking, with increasing focus on circuit adaptations underlying these behaviors. This chapter will discuss the acute and chronic presynaptic effects of ethanol in the CNS, as well as some of the consequences of these effects in amygdala and corticostriatal circuits that are related to excessive seeking/drinking and ethanol abuse.
Keywords: Addiction; Alcohol; Amygdala; Cortex; Endocannabinoid; GABA; Glutamate; Long-term depression; Striatum; Synaptic plasticity; Synaptic transmission.
Figures

Similar articles
-
Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala.Neuropharmacology. 2009 Apr;56(5):886-95. doi: 10.1016/j.neuropharm.2009.01.013. Epub 2009 Jan 21. Neuropharmacology. 2009. PMID: 19371578 Free PMC article.
-
Mu Receptors.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31855381 Free Books & Documents.
-
Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala.J Neurophysiol. 2006 Jul;96(1):433-41. doi: 10.1152/jn.01380.2005. Epub 2006 Apr 19. J Neurophysiol. 2006. PMID: 16624993
-
Synaptic effects induced by alcohol.Curr Top Behav Neurosci. 2013;13:31-86. doi: 10.1007/7854_2011_143. Curr Top Behav Neurosci. 2013. PMID: 21786203 Free PMC article. Review.
-
Synaptic targets: Chronic alcohol actions.Neuropharmacology. 2017 Aug 1;122:85-99. doi: 10.1016/j.neuropharm.2017.01.013. Epub 2017 Jan 17. Neuropharmacology. 2017. PMID: 28108359 Free PMC article. Review.
Cited by
-
Differential sensitivity of human neurons carrying μ opioid receptor (MOR) N40D variants in response to ethanol.Alcohol. 2020 Sep;87:97-109. doi: 10.1016/j.alcohol.2020.05.004. Epub 2020 Jun 17. Alcohol. 2020. PMID: 32561311 Free PMC article.
-
Alcohol Enhances Responses to High Frequency Stimulation in Hippocampus from Transgenic Mice with Increased Astrocyte Expression of IL-6.Cell Mol Neurobiol. 2021 Aug;41(6):1299-1310. doi: 10.1007/s10571-020-00902-6. Epub 2020 Jun 19. Cell Mol Neurobiol. 2021. PMID: 32562098 Free PMC article.
-
Cross-Species Alterations in Synaptic Dopamine Regulation After Chronic Alcohol Exposure.Handb Exp Pharmacol. 2018;248:213-238. doi: 10.1007/164_2018_106. Handb Exp Pharmacol. 2018. PMID: 29675581 Free PMC article. Review.
-
Adaptor protein complex 2 in the orbitofrontal cortex predicts alcohol use disorder.Mol Psychiatry. 2023 Nov;28(11):4766-4776. doi: 10.1038/s41380-023-02236-3. Epub 2023 Sep 7. Mol Psychiatry. 2023. PMID: 37679472 Free PMC article.
-
5. Collaborative Study on the Genetics of Alcoholism: Functional genomics.Genes Brain Behav. 2023 Oct;22(5):e12855. doi: 10.1111/gbb.12855. Epub 2023 Aug 2. Genes Brain Behav. 2023. PMID: 37533187 Free PMC article. Review.
References
-
- Adermark L, Clarke RB, Soderpalm B, Ericson M (2011a). Ethanol-induced modulation of synaptic output from the dorsolateral striatum in rat is regulated by cholinergic interneurons. Neurochem Int 58:693–699 - PubMed
-
- Adermark L, Jonsson S, Ericson M, Soderpalm B (2011b). Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology 61:1160–1165 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

