Environment-transformable sequence-structure relationship: a general mechanism for proteotoxicity
- PMID: 29204881
- PMCID: PMC5899728
- DOI: 10.1007/s12551-017-0369-0
Environment-transformable sequence-structure relationship: a general mechanism for proteotoxicity
Abstract
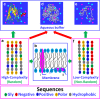
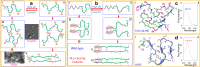
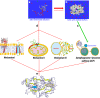
In his Nobel Lecture, Anfinsen stated "the native conformation is determined by the totality of interatomic interactions and hence by the amino acid sequence, in a given environment." As aqueous solutions and membrane systems co-exist in cells, proteins are classified into membrane and non-membrane proteins, but whether one can transform one into the other remains unknown. Intriguingly, many well-folded non-membrane proteins are converted into "insoluble" and toxic forms by aging- or disease-associated factors, but the underlying mechanisms remain elusive. In 2005, we discovered a previously unknown regime of proteins seemingly inconsistent with the classic "Salting-in" dogma: "insoluble" proteins including the integral membrane fragments could be solubilized in the ion-minimized water. We have thus successfully studied "insoluble" forms of ALS-causing P56S-MSP, L126Z-SOD1, nascent SOD1 and C71G-Profilin1, as well as E. coli S1 fragments. The results revealed that these "insoluble" forms are either unfolded or co-exist with their unfolded states. Most unexpectedly, these unfolded states acquire a novel capacity of interacting with membranes energetically driven by the formation of helices/loops over amphiphilic/hydrophobic regions which universally exit in proteins but are normally locked away in their folded native states. Our studies suggest that most, if not all, proteins contain segments which have the dual ability to fold into distinctive structures in aqueous and membrane environments. The abnormal membrane interaction might initiate disease and/or aging processes; and its further coupling with protein aggregation could result in radical proteotoxicity by forming inclusions composed of damaged membranous organelles and protein aggregates. Therefore, environment-transformable sequence-structure relationship may represent a general mechanism for proteotoxicity.
Keywords: Aging; Liquid–liquid phase separation (LLPS); Membrane interaction; Neurodegenerative diseases; Prion-like domains; Proteotoxicity.
Conflict of interest statement
Conflict of interest
Jianxing Song declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Figures


References
-
- Bodner RA, Outeiro TF, Altmann S, Maxwell MM, Cho SH, Hyman BT, McLean PJ, Young AB, Housman DE, Kazantsev AG. Pharmacological promotion of inclusion formation: a therapeutic approach for Huntington’s and Parkinson’s diseases. Proc Natl Acad Sci U S A. 2006;103:4246–4251. doi: 10.1073/pnas.0511256103. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

