Molecular evolution of an oligomeric biocatalyst functioning in lysine biosynthesis
- PMID: 29204887
- PMCID: PMC5899710
- DOI: 10.1007/s12551-017-0350-y
Molecular evolution of an oligomeric biocatalyst functioning in lysine biosynthesis
Abstract
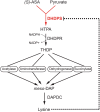
Dihydrodipicolinate synthase (DHDPS) is critical to the production of lysine through the diaminopimelate (DAP) pathway. Elucidation of the function, regulation and structure of this key class I aldolase has been the focus of considerable study in recent years, given that the dapA gene encoding DHDPS has been found to be essential to bacteria and plants. Allosteric inhibition by lysine is observed for DHDPS from plants and some bacterial species, the latter requiring a histidine or glutamate at position 56 (Escherichia coli numbering) over a basic amino acid. Structurally, two DHDPS monomers form the active site, which binds pyruvate and (S)-aspartate β-semialdehyde, with most dimers further dimerising to form a tetrameric arrangement around a solvent-filled centre cavity. The architecture and behaviour of these dimer-of-dimers is explored in detail, including biophysical studies utilising analytical ultracentrifugation, small-angle X-ray scattering and macromolecular crystallography that show bacterial DHDPS tetramers adopt a head-to-head quaternary structure, compared to the back-to-back arrangement observed for plant DHDPS enzymes. Finally, the potential role of pyruvate in providing substrate-mediated stabilisation of DHDPS is considered.
Keywords: Allostery; Antibiotic; Crystal; Herbicide; SAXS; Sedimentation.
Conflict of interest statement
Conflict of interest
Tatiana P. Soares da Costa declares that she has no conflict of interest. Belinda M. Abbott declares that she has no conflict of interest. Anthony R. Gendall declares that he has no conflict of interest. Santosh Panjikar declares that he has no conflict of interest. Matthew A. Perugini declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures
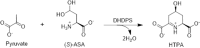


Similar articles
-
Characterization of recombinant dihydrodipicolinate synthase from the bread wheat Triticum aestivum.Planta. 2018 Aug;248(2):381-391. doi: 10.1007/s00425-018-2894-x. Epub 2018 May 9. Planta. 2018. PMID: 29744651
-
Crystal, solution and in silico structural studies of dihydrodipicolinate synthase from the common grapevine.PLoS One. 2012;7(6):e38318. doi: 10.1371/journal.pone.0038318. Epub 2012 Jun 25. PLoS One. 2012. PMID: 22761676 Free PMC article.
-
Structure and evolution of a novel dimeric enzyme from a clinically important bacterial pathogen.J Biol Chem. 2008 Oct 10;283(41):27598-27603. doi: 10.1074/jbc.M804231200. Epub 2008 Aug 5. J Biol Chem. 2008. PMID: 18684709
-
Dihydrodipicolinate Synthase: Structure, Dynamics, Function, and Evolution.Subcell Biochem. 2017;83:271-289. doi: 10.1007/978-3-319-46503-6_10. Subcell Biochem. 2017. PMID: 28271480 Review.
-
Feedback inhibition of dihydrodipicolinate synthase enzymes by L-lysine.Biol Chem. 1997 Mar-Apr;378(3-4):207-10. doi: 10.1515/bchm.1997.378.3-4.207. Biol Chem. 1997. PMID: 9165072 Review.
Cited by
-
A dual-target herbicidal inhibitor of lysine biosynthesis.Elife. 2022 Jun 20;11:e78235. doi: 10.7554/eLife.78235. Elife. 2022. PMID: 35723913 Free PMC article.
-
Creating Single-Cell Protein-Producing Bacillus subtilis Mutants Using Chemical Mutagen and Amino Acid Inhibitors.Scientifica (Cairo). 2024 Nov 29;2024:8968295. doi: 10.1155/sci5/8968295. eCollection 2024. Scientifica (Cairo). 2024. PMID: 39649941 Free PMC article.
-
Characterization of recombinant dihydrodipicolinate synthase from the bread wheat Triticum aestivum.Planta. 2018 Aug;248(2):381-391. doi: 10.1007/s00425-018-2894-x. Epub 2018 May 9. Planta. 2018. PMID: 29744651
-
Towards novel herbicide modes of action by inhibiting lysine biosynthesis in plants.Elife. 2021 Jul 27;10:e69444. doi: 10.7554/eLife.69444. Elife. 2021. PMID: 34313586 Free PMC article.
-
Repurposed inhibitor of bacterial dihydrodipicolinate reductase exhibits effective herbicidal activity.Commun Biol. 2023 May 22;6(1):550. doi: 10.1038/s42003-023-04895-y. Commun Biol. 2023. PMID: 37217566 Free PMC article.
References
-
- Atkinson SC, Dogovski C, Newman J, Dobson RC, Perugini MA. Cloning, expression, purification and crystallization of dihydrodipicolinate synthase from the grapevine Vitis vinifera. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011;67:1537–1541. doi: 10.1107/S1744309111038395. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

