Potential for thermal damage to the blood-brain barrier during craniotomy: implications for intracortical recording microelectrodes
- PMID: 29205169
- PMCID: PMC6482047
- DOI: 10.1088/1741-2552/aa9f32
Potential for thermal damage to the blood-brain barrier during craniotomy: implications for intracortical recording microelectrodes
Abstract
Objective: Our objective was to determine how readily disruption of the blood-brain barrier (BBB) occurred as a result of bone drilling during a craniotomy to implant microelectrodes in rat cortex. While the phenomenon of heat production during bone drilling is well known, practices to evade damage to the underlying brain tissue are inconsistently practiced and reported in the literature.
Approach: We conducted a review of the intracortical microelectrode literature to summarize typical approaches to mitigate drill heating during rodent craniotomies. Post mortem skull-surface and transient brain-surface temperatures were experimentally recorded using an infrared camera and thermocouple, respectively. A number of drilling conditions were tested, including varying drill speed and continuous versus intermittent contact. In vivo BBB permeability was assayed 1 h after the craniotomy procedure using Evans blue dye.
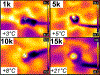
Main results: Of the reviewed papers that mentioned methods to mitigate thermal damage during craniotomy, saline irrigation was the most frequently cited (in six of seven papers). In post mortem tissues, we observed increases in skull-surface temperature ranging from +3 °C to +21 °C, dependent on drill speed. In vivo, pulsed-drilling (2 s-on/2 s-off) and slow-drilling speeds (1000 r.p.m.) were the most effective methods we studied to mitigate heating effects from drilling, while inconclusive results were obtained with saline irrigation.
Significance: Neuroinflammation, initiated by damage to the BBB and perpetuated by the foreign body response, is thought to play a key role in premature failure of intracortical recording microelectrodes. This study demonstrates the extreme sensitivity of the BBB to overheating caused by bone drilling. To avoid damage to the BBB, the authors recommend that craniotomies be drilled with slow speeds and/or with intermittent drilling with complete removal of the drill from the skull during 'off' periods. While saline alone was ineffective at preventing overheating, its use is still recommended to remove bone dust from the surgical site and to augment other cooling methods.
Conflict of interest statement
Conflict of interest statement:
None of the authors have a conflict of interest
Figures
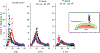
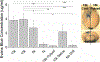
Similar articles
-
Assessment of Thermal Damage from Robot-Drilled Craniotomy for Cranial Window Surgery in Mice.J Vis Exp. 2022 Nov 11;(189). doi: 10.3791/64188. J Vis Exp. 2022. PMID: 36440896
-
Slow drilling speeds for single-drill implant bed preparation. Experimental in vitro study.Clin Oral Investig. 2018 Jan;22(1):349-359. doi: 10.1007/s00784-017-2119-x. Epub 2017 Apr 22. Clin Oral Investig. 2018. PMID: 28434045
-
Excessive Heat Generation by Power-Driven Craniotomy Tools: A Possible Cause of Autologous Bone Flap Resorption Observed in an Ex Vivo Simulation.World Neurosurg. 2024 Jul;187:e914-e919. doi: 10.1016/j.wneu.2024.05.010. Epub 2024 May 9. World Neurosurg. 2024. PMID: 38734170
-
Understanding the Role of Innate Immunity in the Response to Intracortical Microelectrodes.Crit Rev Biomed Eng. 2018;46(4):341-367. doi: 10.1615/CritRevBiomedEng.2018027166. Crit Rev Biomed Eng. 2018. PMID: 30806249 Free PMC article. Review.
-
Surgical Drill Bit Design and Thermomechanical Damage in Bone Drilling: A Review.Ann Biomed Eng. 2021 Jan;49(1):29-56. doi: 10.1007/s10439-020-02600-2. Epub 2020 Aug 28. Ann Biomed Eng. 2021. PMID: 32860111 Review.
Cited by
-
Fabrication Methods and Chronic In Vivo Validation of Mechanically Adaptive Microfluidic Intracortical Devices.Micromachines (Basel). 2023 May 9;14(5):1015. doi: 10.3390/mi14051015. Micromachines (Basel). 2023. PMID: 37241639 Free PMC article.
-
Effects of iron accumulation and its chelation on oxidative stress in intracortical implants.Acta Biomater. 2025 Jun 15;200:703-723. doi: 10.1016/j.actbio.2025.05.026. Epub 2025 May 10. Acta Biomater. 2025. PMID: 40355018
-
Inhibition of the cluster of differentiation 14 innate immunity pathway with IAXO-101 improves chronic microelectrode performance.J Neural Eng. 2018 Apr;15(2):025002. doi: 10.1088/1741-2552/aaa03e. J Neural Eng. 2018. PMID: 29219114 Free PMC article.
-
Interfacing with the Brain: How Nanotechnology Can Contribute.ACS Nano. 2025 Mar 25;19(11):10630-10717. doi: 10.1021/acsnano.4c10525. Epub 2025 Mar 10. ACS Nano. 2025. PMID: 40063703 Free PMC article. Review.
-
Transcranial chronic optical access to longitudinally measure cerebral blood flow.J Neurosci Methods. 2021 Feb 15;350:109044. doi: 10.1016/j.jneumeth.2020.109044. Epub 2020 Dec 16. J Neurosci Methods. 2021. PMID: 33340556 Free PMC article.
References
-
- Donoghue JP. Connecting cortex to machines: recent advances in brain interfaces. Nature neuroscience 2002;5(Supp):1085–8. - PubMed
-
- Peckham PH, Knutson JS. Functional Electrical Stimulation for Neuromuscular Applications. Annu Rev Biomed Eng 2005;7(1):327–60. - PubMed
-
- Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 2016. April 13;533(7602):247–50. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
