Roadmap on semiconductor-cell biointerfaces
- PMID: 29205173
- PMCID: PMC6599646
- DOI: 10.1088/1478-3975/aa9f34
Roadmap on semiconductor-cell biointerfaces
Abstract
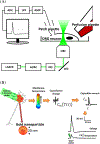
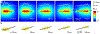
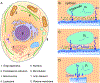
This roadmap outlines the role semiconductor-based materials play in understanding the complex biophysical dynamics at multiple length scales, as well as the design and implementation of next-generation electronic, optoelectronic, and mechanical devices for biointerfaces. The roadmap emphasizes the advantages of semiconductor building blocks in interfacing, monitoring, and manipulating the activity of biological components, and discusses the possibility of using active semiconductor-cell interfaces for discovering new signaling processes in the biological world.
Figures














References
-
- Irimia-Vladu M et al. 2010. Biocompatible and biodegradable materials for organic field-effect transistors Adv. Funct. Mater 20 4069–76
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
