Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04)
- PMID: 29205286
- PMCID: PMC5814824
- DOI: 10.1002/cncr.31128
Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04)
Abstract
Background: Cachexia, described as weight loss (mainly in lean body mass [LBM]) and anorexia, is common in patients with advanced cancer. This study examined the efficacy and safety of anamorelin (ONO-7643), a novel selective ghrelin receptor agonist, in Japanese cancer patients with cachexia.
Methods: This double-blind clinical trial (ONO-7643-04) enrolled 174 patients with unresectable stage III/IV non-small cell lung cancer (NSCLC) and cachexia in Japan. Patients were randomized to daily oral anamorelin (100 mg) or a placebo for 12 weeks. The primary endpoint was the change from the baseline LBM (measured with dual-energy x-ray absorptiometry) over 12 weeks. The secondary endpoints were changes in appetite, body weight, quality of life, handgrip strength (HGS), and 6-minute walk test (6MWT) results.
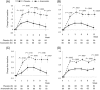
Results: The least squares mean change (plus or minus the standard error) in LBM from the baseline over 12 weeks was 1.38 ± 0.18 and -0.17 ± 0.17 kg in the anamorelin and placebo groups, respectively (P < .0001). Changes from the baseline in LBM, body weight, and anorexia symptoms showed significant differences between the 2 treatment groups at all time points. Anamorelin increased prealbumin at weeks 3 and 9. No changes in HGS or 6MWT were detected between the groups. Twelve weeks' treatment with anamorelin was safe and well tolerated in NSCLC patients.
Conclusions: Anamorelin significantly increased LBM and improved anorexia symptoms and the nutritional state, but not motor function, in Japanese patients with advanced NSCLC. Because no effective treatment for cancer cachexia is currently available, anamorelin can be a beneficial treatment option. Cancer 2018;124:606-16. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
Keywords: anamorelin (ONO-7643); cachexia; lean body mass; non-small cell lung cancer; randomized controlled trial.
© 2017 American Cancer Society.
Figures


Comment in
-
Cancer cachexia: Are we ready to take a step forward?Cancer. 2018 Feb 1;124(3):456-458. doi: 10.1002/cncr.31126. Epub 2017 Dec 4. Cancer. 2018. PMID: 29205293 No abstract available.
-
Palliative care: Anamorelin provides benefit to patients with cachexia.Nat Rev Clin Oncol. 2018 Feb;15(2):68. doi: 10.1038/nrclinonc.2017.204. Epub 2017 Dec 19. Nat Rev Clin Oncol. 2018. PMID: 29255237 No abstract available.
References
-
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491‐497. - PubMed
-
- Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med. 1988;148:1586‐1591. - PubMed
-
- von Haehling S, Anker SD. Treatment of cachexia: an overview of recent developments. J Am Med Dir Assoc. 2014;15:866‐872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

