Quantitative analysis of hepatic macro- and microvascular alterations during cirrhogenesis in the rat
- PMID: 29205328
- PMCID: PMC5807949
- DOI: 10.1111/joa.12760
Quantitative analysis of hepatic macro- and microvascular alterations during cirrhogenesis in the rat
Abstract
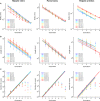
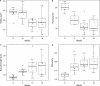
Cirrhosis represents the end-stage of any persistent chronically active liver disease. It is characterized by the complete replacement of normal liver tissue by fibrosis, regenerative nodules, and complete fibrotic vascularized septa. The resulting angioarchitectural distortion contributes to an increasing intrahepatic vascular resistance, impeding liver perfusion and leading to portal hypertension. To date, knowledge on the dynamically evolving pathological changes of the hepatic vasculature during cirrhogenesis remains limited. More specifically, detailed anatomical data on the vascular adaptations during disease development is lacking. To address this need, we studied the 3D architecture of the hepatic vasculature during induction of cirrhogenesis in a rat model. Cirrhosis was chemically induced with thioacetamide (TAA). At predefined time points, the hepatic vasculature was fixed and visualized using a combination of vascular corrosion casting and deep tissue microscopy. Three-dimensional reconstruction and data-fitting enabled cirrhogenic features to extracted at multiple scales, portraying the impact of cirrhosis on the hepatic vasculature. At the macrolevel, we noticed that regenerative nodules severely compressed pliant venous vessels from 12 weeks of TAA intoxication onwards. Especially hepatic veins were highly affected by this compression, with collapsed vessel segments severely reducing perfusion capabilities. At the microlevel, we discovered zone-specific sinusoidal degeneration, with sinusoids located near the surface being more affected than those in the middle of a liver lobe. Our data shed light on and quantify the evolving angioarchitecture during cirrhogenesis. These findings may prove helpful for future targeted invasive interventions.
Keywords: 3D reconstruction; cirrhosis; deep tissue microscopy; hepatic vasculature; micro-CT imaging; morphological analysis; rat liver; vascular corrosion casting.
© 2017 Anatomical Society.
Figures





References
-
- Aagaard J, Jensen LI, Sorensen T, et al. (1982) Recanalized umbilical vein in portal hypertension. Am J Roentgenol 139, 1107–1110. - PubMed
-
- Alexander B, Cottam H, Naftalin R (2001) Hepatic arterial perfusion regulates portal venous flow between hepatic sinusoids and intrahepatic shunts in the normal rat liver in vitro . Pflugers Arch 443, 257–264. - PubMed
-
- Bodner G, Peer S, Karner M, et al. (2002) Nontumorous vascular malformations in the liver: color Doppler ultrasonographic findings. J Ultrasound Med 21, 187–197. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

