VapA of Rhodococcus equi binds phosphatidic acid
- PMID: 29205554
- PMCID: PMC5777868
- DOI: 10.1111/mmi.13892
VapA of Rhodococcus equi binds phosphatidic acid
Abstract
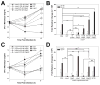
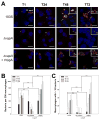
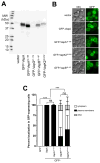
Rhodococcus equi is a multihost, facultative intracellular bacterial pathogen that primarily causes pneumonia in foals less than six months in age and immunocompromised people. Previous studies determined that the major virulence determinant of R. equi is the surface bound virulence associated protein A (VapA). The presence of VapA inhibits the maturation of R. equi-containing phagosomes and promotes intracellular bacterial survival, as determined by the inability of vapA deletion mutants to replicate in host macrophages. While the mechanism of action of VapA remains elusive, we show that soluble recombinant VapA32-189 both rescues the intramacrophage replication defect of a wild type R. equi strain lacking the vapA gene and enhances the persistence of nonpathogenic Escherichia coli in macrophages. During macrophage infection, VapA was observed at both the bacterial surface and at the membrane of the host-derived R. equi containing vacuole, thus providing an opportunity for VapA to interact with host constituents and promote alterations in phagolysosomal function. In support of the observed host membrane binding activity of VapA, we also found that rVapA32-189 interacted specifically with liposomes containing phosphatidic acid in vitro. Collectively, these data demonstrate a lipid binding property of VapA, which may be required for its function during intracellular infection.
© 2017 John Wiley & Sons Ltd.
Figures
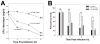
Similar articles
-
Virulence-associated protein A from Rhodococcus equi is an intercompartmental pH-neutralising virulence factor.Cell Microbiol. 2019 Jan;21(1):e12958. doi: 10.1111/cmi.12958. Epub 2018 Nov 9. Cell Microbiol. 2019. PMID: 30251327
-
Deletion of vapA encoding Virulence Associated Protein A attenuates the intracellular actinomycete Rhodococcus equi.Mol Microbiol. 2003 Oct;50(1):115-28. doi: 10.1046/j.1365-2958.2003.03689.x. Mol Microbiol. 2003. PMID: 14507368
-
Specific preadaptations of Rhodococcus equi cooperate with its Virulence-associated protein A during macrophage infection.Mol Microbiol. 2023 Mar;119(3):285-301. doi: 10.1111/mmi.15026. Epub 2023 Jan 16. Mol Microbiol. 2023. PMID: 36627747
-
Pathogenesis and virulence of Rhodococcus equi.Vet Microbiol. 1997 Jun 16;56(3-4):257-68. doi: 10.1016/s0378-1135(97)00094-1. Vet Microbiol. 1997. PMID: 9226840 Review.
-
Molecular and infection biology of the horse pathogen Rhodococcus equi.FEMS Microbiol Rev. 2009 Sep;33(5):870-91. doi: 10.1111/j.1574-6976.2009.00181.x. Epub 2009 Apr 23. FEMS Microbiol Rev. 2009. PMID: 19453748 Review.
Cited by
-
Protective immune response against Rhodococcus equi: An innate immunity-focused review.Equine Vet J. 2025 May;57(3):563-586. doi: 10.1111/evj.14214. Epub 2024 Sep 11. Equine Vet J. 2025. PMID: 39258739 Free PMC article. Review.
-
Impact of surface receptors TLR2, CR3, and FcγRIII on Rhodococcus equi phagocytosis and intracellular survival in macrophages.Infect Immun. 2024 Jan 16;92(1):e0038323. doi: 10.1128/iai.00383-23. Epub 2023 Nov 29. Infect Immun. 2024. PMID: 38018994 Free PMC article.
-
The N-terminal domain is required for cell surface localisation of VapA, a member of the Vap family of Rhodococcus equi virulence proteins.PLoS One. 2024 Feb 29;19(2):e0298900. doi: 10.1371/journal.pone.0298900. eCollection 2024. PLoS One. 2024. PMID: 38421980 Free PMC article.
-
Effect of Macrolide and Rifampin Resistance on the Fitness of Rhodococcus equi.Appl Environ Microbiol. 2019 Mar 22;85(7):e02665-18. doi: 10.1128/AEM.02665-18. Print 2019 Apr 1. Appl Environ Microbiol. 2019. PMID: 30683740 Free PMC article.
-
Multi-host distribution of Rhodococcus equi (Prescottella equi) strains and their phylogenomic clustering.BMC Microbiol. 2025 Jul 21;25(1):447. doi: 10.1186/s12866-025-04152-8. BMC Microbiol. 2025. PMID: 40691552 Free PMC article.
References
-
- Barton MD, Hughes KL. Ecology of Rhodococcus equi. Vet Microbiol. 1984;9:65–76. - PubMed
-
- Benoit S, Benachour A, Taouji S, Auffray Y, Hartke A. Induction of vap genes encoded by the virulence plasmid of Rhodococcus equi during acid tolerance response. Res Microbiol. 2001;152:439–449. - PubMed
-
- Burd CG, Emr SD. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;2:157–162. - PubMed
-
- Burton AJ, Giguere S, Berghaus LJ, Hondalus MK, Arnold RD. Efficacy of liposomal gentamicin against Rhodococcus equi in a mouse infection model and colocalization with R. equi in equine alveolar macrophages. Vet Microbiol. 2015;176:292–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

