Conformational dynamics in TRPV1 channels reported by an encoded coumarin amino acid
- PMID: 29206105
- PMCID: PMC5716661
- DOI: 10.7554/eLife.28626
Conformational dynamics in TRPV1 channels reported by an encoded coumarin amino acid
Abstract
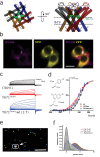
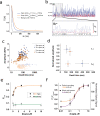
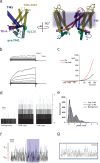
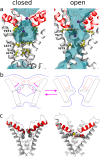
TRPV1 channels support the detection of noxious and nociceptive input. Currently available functional and structural data suggest that TRPV1 channels have two gates within their permeation pathway: one formed by a 'bundle-crossing' at the intracellular entrance and a second constriction at the selectivity filter. To describe conformational changes associated with channel gating, the fluorescent non-canonical amino acid coumarin-tyrosine was genetically encoded at Y671, a residue proximal to the selectivity filter. Total internal reflection fluorescence microscopy was performed to image the conformational dynamics of the channels in live cells. Photon counts and optical fluctuations from coumarin encoded within TRPV1 tetramers correlates with channel activation by capsaicin, providing an optical marker of conformational dynamics at the selectivity filter. In agreement with the fluorescence data, molecular dynamics simulations display alternating solvent exposure of Y671 in the closed and open states. Overall, the data point to a dynamic selectivity filter that may serve as a gate for permeation.
Keywords: SPAD; TRPV1; biophysics; computational biology; coumarin; gating; non-canonical amino acids; none; pore; structural biology; systems biology.
Plain language summary
Cells use proteins on their surface as sensors to help them to assess and explore their environments and adapt to external conditions. The transient receptor potential (TRP) ion channels form one such family of proteins. Sodium, potassium and calcium ions can move through TRP channels to enter and exit cells, and by doing so trigger changes in the cell that help it respond to an external stimulus. The channels have physical “gates” that can open and close to control the flow of the ions. When the TRP channel detects a stimulus – which could take the form of specific chemicals, or a change in temperature, pressure or voltage – it changes shape, causing the gate to open. Researchers have a number of unanswered questions about how TRP channels work. Where in the channels are gates located? How do the channels control the flow of ions, and how do they communicate with each other? And which regions of the protein sense environmental cues? As a result, new technologies are being developed to make it easier to study the types of rearrangements that TRP channels experience when they activate. Steinberg, Kasimova et al. have used total internal reflection microscopy – a method that images fluorescent molecules – to measure the conformational change of a single TRP channel in a living cell. This channel, called TRPV1, senses external heat and plays an important role in pain perception. Its gate can also be opened by the pungent compound of chili pepper, capsaicin. The results of the experiments suggest that a selectivity filter region in TRPV1 channels changes its shape when the channel opens in response to capsaicin. Then, this selectivity filter appears to do double duty – it controls which types of ions pass through the channels as well as controlling their flow rate. Because of its role in pain perception, having a better understanding of how TRPV1 works will be valuable for researchers who are trying to develop new pain relief treatments. The so-called ‘seeing is believing’ method used by Steinberg, Kasimova et al. could also be used to study other membrane proteins, both to guide drug development and to improve our understanding of how cells interact with their environment.
Conflict of interest statement
No competing interests declared.
Figures






Similar articles
-
Toward elucidating the heat activation mechanism of the TRPV1 channel gating by molecular dynamics simulation.Proteins. 2016 Dec;84(12):1938-1949. doi: 10.1002/prot.25177. Epub 2016 Oct 24. Proteins. 2016. PMID: 27699868 Free PMC article.
-
Permeation and dynamics of an open-activated TRPV1 channel.J Mol Biol. 2015 Jan 30;427(2):537-49. doi: 10.1016/j.jmb.2014.11.016. Epub 2014 Dec 3. J Mol Biol. 2015. PMID: 25479373
-
The ion selectivity filter is not an activation gate in TRPV1-3 channels.Elife. 2019 Nov 14;8:e51212. doi: 10.7554/eLife.51212. Elife. 2019. PMID: 31724952 Free PMC article.
-
TRPV1 Receptors and Signal Transduction.In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 5. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 5. PMID: 21204507 Free Books & Documents. Review.
-
ThermoTRP channels as modular proteins with allosteric gating.Cell Calcium. 2007 Oct-Nov;42(4-5):427-38. doi: 10.1016/j.ceca.2007.04.004. Epub 2007 May 17. Cell Calcium. 2007. PMID: 17499848 Review.
Cited by
-
TRPV1 pore turret dictates distinct DkTx and capsaicin gating.Proc Natl Acad Sci U S A. 2018 Dec 11;115(50):E11837-E11846. doi: 10.1073/pnas.1809662115. Epub 2018 Nov 21. Proc Natl Acad Sci U S A. 2018. PMID: 30463948 Free PMC article.
-
Implications of a temperature-dependent heat capacity for temperature-gated ion channels.Proc Natl Acad Sci U S A. 2023 Jun 13;120(24):e2301528120. doi: 10.1073/pnas.2301528120. Epub 2023 Jun 6. Proc Natl Acad Sci U S A. 2023. PMID: 37279277 Free PMC article.
-
Real-Time Observation of Polymer Fluctuations During Phase Transition Using Transmission Electron Microscope.Polymers (Basel). 2025 Jan 23;17(3):292. doi: 10.3390/polym17030292. Polymers (Basel). 2025. PMID: 39940500 Free PMC article.
-
A rationally designed orthogonal synthetase for genetically encoded fluorescent amino acids.Heliyon. 2020 Oct 7;6(10):e05140. doi: 10.1016/j.heliyon.2020.e05140. eCollection 2020 Oct. Heliyon. 2020. PMID: 33083608 Free PMC article.
-
Ion Channel Sensing: Are Fluctuations the Crux of the Matter?J Phys Chem Lett. 2018 Mar 15;9(6):1260-1264. doi: 10.1021/acs.jpclett.7b03396. Epub 2018 Feb 27. J Phys Chem Lett. 2018. PMID: 29439562 Free PMC article.
References
-
- Bronzi D, Villa F, Tisa S, Tosi A, Zappa F, Durini D, Weyers S, Brockherde W. 100 000 Frames/s 64 × 32 Single-Photon Detector Array for 2-D Imaging and 3-D Ranging. IEEE Journal of Selected Topics in Quantum Electronics. 2014;20:354–363. doi: 10.1109/JSTQE.2014.2341562. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

