One year follow-up after a randomized controlled trial of a 130 g/day low-carbohydrate diet in patients with type 2 diabetes mellitus and poor glycemic control
- PMID: 29206237
- PMCID: PMC5714344
- DOI: 10.1371/journal.pone.0188892
One year follow-up after a randomized controlled trial of a 130 g/day low-carbohydrate diet in patients with type 2 diabetes mellitus and poor glycemic control
Abstract
Background & aims: Recently, we conducted a prospective randomized controlled trial (RCT) showing that a 6-month 130g/day low-carbohydrate diet (LCD) reduced HbA1c and BMI more than a calorie restricted diet (CRD). [1] To assess whether the benefits of the LCD persisted after the intensive intervention, we compared HbA1c and BMI between the LCD and CRD groups at 1 year after the end of the 6-month RCT.
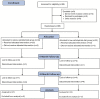
Methods: Following the end of the 6-month RCT, patients were allowed to manage their own diets with periodic outpatient visits. One year later, we analyzed clinical and nutrition data.
Results: Of the 66 participants in the original study, 27 in the CRD group and 22 in the LCD group completed this trial. One year after the end of the original RCT, the carbohydrate intake was comparable between the groups (215 [189-243]/day in the CRD group and 214 (176-262) g/day in the LCD group). Compared with the baseline data, HbA1c and BMI were decreased in both groups (CRD: HbA1c -0.4 [-0.9 to 0.3] % and BMI -0.63 [-1.20 to 0.18] kg/m2; LCD: HbA1c -0.35 [-1.0 to 0.35] % and BMI -0.77 [-1.15 to -0.12] kg/m2). There were no significant differences in HbA1c and BMI between the groups.
Conclusions: One year after the diet therapy intervention, the beneficial effect of the LCD on reduction of HbA1c and BMI did not persist in comparison with CRD. However, combining the data of both groups, significant improvements in HbA1c and BMI from baseline were observed. Although the superiority of the LCD disappeared 1 year after the intensive intervention, these data suggest that well-constructed nutrition therapy programs, both CRD and LCD, were equally effective in improving HbA1c for at least 1 year.
Trial registration: University Hospital Medical Information Network (UMIN) ID000010663.
Conflict of interest statement
Figures
References
-
- Sato J, Kanazawa A, Makita S, Hatae C, Komiya K, Shimizu T, et al. A randomized controlled trial of 130 g/day low-carbohydrate diet in type 2 diabetes with poor glycemic control. Clinical nutrition (Edinburgh, Scotland). 2016. Epub 2016/07/31. doi: 10.1016/j.clnu.2016.07.003 . - DOI - PubMed
-
- Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140(10):778–85. Epub 2004/05/19. . - PubMed
-
- Davis NJ, Tomuta N, Schechter C, Isasi CR, Segal-Isaacson CJ, Stein D, et al. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care. 2009;32(7):1147–52. Epub 2009/04/16. doi: 10.2337/dc08-2108 . - DOI - PMC - PubMed
-
- Guldbrand H, Dizdar B, Bunjaku B, Lindstrom T, Bachrach-Lindstrom M, Fredrikson M, et al. In type 2 diabetes, randomisation to advice to follow a low-carbohydrate diet transiently improves glycaemic control compared with advice to follow a low-fat diet producing a similar weight loss. Diabetologia. 2012;55(8):2118–27. Epub 2012/05/09. doi: 10.1007/s00125-012-2567-4 . - DOI - PMC - PubMed
-
- Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. 2014;161(5):309–18. Epub 2014/09/03. doi: 10.7326/M14-0180 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical