Restoration of the prolyl-hydroxylase domain protein-3 oxygen-sensing mechanism is responsible for regulation of HIF2α expression and induction of sensitivity of myeloma cells to hypoxia-mediated apoptosis
- PMID: 29206844
- PMCID: PMC5716583
- DOI: 10.1371/journal.pone.0188438
Restoration of the prolyl-hydroxylase domain protein-3 oxygen-sensing mechanism is responsible for regulation of HIF2α expression and induction of sensitivity of myeloma cells to hypoxia-mediated apoptosis
Abstract
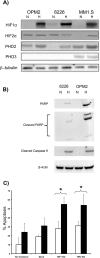
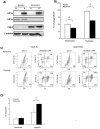
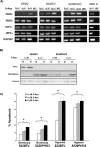
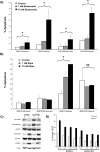
Multiple myeloma (MM) is an incurable disease of malignant plasma B-cells that infiltrate the bone marrow (BM), resulting in bone destruction, anemia, renal impairment and infections. Physiologically, the BM microenvironment is hypoxic and this promotes MM progression and contributes to resistance to chemotherapy. Since aberrant hypoxic responses may result in the selection of more aggressive tumor phenotypes, we hypothesized that targeting the hypoxia-inducible factor (HIF) pathways will be an effective anti-MM therapeutic strategy. We demonstrated that MM cells are resistant to hypoxia-mediated apoptosis in vivo and in vitro, and that constitutive expression of HIF2α contributed to this resistance. Since epigenetic silencing of the prolyl-hydroxylase-domain-3 (PHD3) enzyme responsible for the O2-dependent regulation of HIF2α is frequently observed in MM tumors, we asked if PHD3 plays a role in regulating sensitivity to hypoxia. We found that restoring PHD3 expression using a lentivirus vector or overcoming PHD3 epigenetic silencing using a demethyltransferase inhibitor, 5-Aza-2'-deoxycytidine (5-Aza-dC), rescued O2-dependent regulation of HIF2α and restored sensitivity of MM cells to hypoxia-mediated apoptosis. This provides a rationale for targeting the PHD3-mediated regulation of the adaptive cellular hypoxic response in MM and suggests that targeting the O2-sensing pathway, alone or in combination with other anti-myeloma chemotherapeutics, may have clinical efficacy.
Conflict of interest statement
Figures
Similar articles
-
Hypoxia-inducible factor (HIF)-prolyl hydroxylase 3 (PHD3) maintains high HIF2A mRNA levels in clear cell renal cell carcinoma.J Biol Chem. 2019 Mar 8;294(10):3760-3771. doi: 10.1074/jbc.RA118.004902. Epub 2019 Jan 7. J Biol Chem. 2019. PMID: 30617181 Free PMC article.
-
A DNA-binding Molecule Targeting the Adaptive Hypoxic Response in Multiple Myeloma Has Potent Antitumor Activity.Mol Cancer Res. 2016 Mar;14(3):253-66. doi: 10.1158/1541-7786.MCR-15-0361. Epub 2016 Jan 22. Mol Cancer Res. 2016. PMID: 26801054 Free PMC article.
-
Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases.Biochem J. 2004 Aug 1;381(Pt 3):761-7. doi: 10.1042/BJ20040620. Biochem J. 2004. PMID: 15104534 Free PMC article.
-
The regulation, localization, and functions of oxygen-sensing prolyl hydroxylase PHD3.Biol Chem. 2013 Apr;394(4):449-57. doi: 10.1515/hsz-2012-0330. Biol Chem. 2013. PMID: 23380539 Review.
-
Impact of hypoxia on the pathogenesis and therapy resistance in multiple myeloma.Cancer Sci. 2021 Oct;112(10):3995-4004. doi: 10.1111/cas.15087. Epub 2021 Aug 9. Cancer Sci. 2021. PMID: 34310776 Free PMC article. Review.
Cited by
-
Loss of Heterozygosity for KrasG12D Promotes Malignant Phenotype of Pancreatic Ductal Adenocarcinoma by Activating HIF-2α-c-Myc-Regulated Glutamine Metabolism.Int J Mol Sci. 2022 Jun 15;23(12):6697. doi: 10.3390/ijms23126697. Int J Mol Sci. 2022. PMID: 35743139 Free PMC article.
-
Acidification of intracellular pH in MM tumor cells overcomes resistance to hypoxia-mediated apoptosis in vitro and in vivo.Front Oncol. 2023 Nov 3;13:1268421. doi: 10.3389/fonc.2023.1268421. eCollection 2023. Front Oncol. 2023. PMID: 38023253 Free PMC article.
-
Can Targeting Hypoxia-Mediated Acidification of the Bone Marrow Microenvironment Kill Myeloma Tumor Cells?Front Oncol. 2021 Jul 19;11:703878. doi: 10.3389/fonc.2021.703878. eCollection 2021. Front Oncol. 2021. PMID: 34350119 Free PMC article.
-
Targeting ERK-Hippo Interplay in Cancer Therapy.Int J Mol Sci. 2020 May 3;21(9):3236. doi: 10.3390/ijms21093236. Int J Mol Sci. 2020. PMID: 32375238 Free PMC article. Review.
References
-
- Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. Embo J. 2012;31(11):2448–60. Epub 2012/05/09. doi: emboj2012125 [pii] doi: 10.1038/emboj.2012.125 ; PubMed Central PMCID: PMC3365421. - DOI - PMC - PubMed
-
- Fandrey J, Gorr TA, Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovascular research. 2006;71(4):642–51. doi: 10.1016/j.cardiores.2006.05.005 . - DOI - PubMed
-
- Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5. Epub 1999/12/03. . - PubMed
-
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365(6):537–47. Epub 2011/08/13. doi: 10.1056/NEJMra1011165 . - DOI - PubMed
-
- Place TL, Domann FE. Prolyl-hydroxylase 3: Evolving Roles for an Ancient Signaling Protein. Hypoxia (Auckl). 2013;2013(1):13–7. doi: 10.2147/HP.S50091 ; PubMed Central PMCID: PMCPMC3963164. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

