Z-disc protein CHAPb induces cardiomyopathy and contractile dysfunction in the postnatal heart
- PMID: 29206857
- PMCID: PMC5716575
- DOI: 10.1371/journal.pone.0189139
Z-disc protein CHAPb induces cardiomyopathy and contractile dysfunction in the postnatal heart
Abstract
Aims: The Z-disc is a crucial structure of the sarcomere and is implicated in mechanosensation/transduction. Dysregulation of Z-disc proteins often result in cardiomyopathy. We have previously shown that the Z-disc protein Cytoskeletal Heart-enriched Actin-associated Protein (CHAP) is essential for cardiac and skeletal muscle development. Furthermore, the CHAP gene has been associated with atrial fibrillation in humans. Here, we studied the misregulated expression of CHAP isoforms in heart disease.
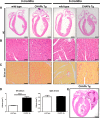
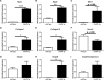
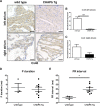
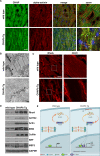
Methods and results: Mice that underwent transverse aortic constriction and calcineurin transgenic (Tg) mice, both models of experimental heart failure, displayed a significant increase in cardiac expression of fetal isoform CHAPb. To investigate whether increased expression of CHAPb postnatally is sufficient to induce cardiomyopathy, we generated CHAPb Tg mice under the control of the cardiac-specific αMHC promoter. CHAPb Tg mice displayed cardiac hypertrophy, interstitial fibrosis and enlargement of the left atrium at three months, which was more pronounced at the age of six months. Hypertrophy and fibrosis were confirmed by evidence of activation of the hypertrophic gene program (Nppa, Nppb, Myh7) and increased collagen expression, respectively. Connexin40 and 43 were downregulated in the left atrium, which was associated with delayed atrioventricular conduction. Tg hearts displayed both systolic and diastolic dysfunction partly caused by impaired sarcomere function evident from a reduced force generating capacity of single cardiomyocytes. This co-incided with activation of the actin signalling pathway leading to the formation of stress fibers.
Conclusion: This study demonstrated that the fetal isoform CHAPb initiates progression towards cardiac hypertrophy, which is accompanied by delayed atrioventricular conduction and diastolic dysfunction. Moreover, CHAP may be a novel therapeutic target or candidate gene for screening in cardiomyopathies and atrial fibrillation.
Conflict of interest statement
Figures


References
-
- Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacological research. 2010;61(4):269–80. doi: 10.1016/j.phrs.2009.11.012 - DOI - PubMed
-
- Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochemical and biophysical research communications. 2004;322(4):1178–91. doi: 10.1016/j.bbrc.2004.07.121 - DOI - PubMed
-
- Kong SW, Hu YW, Ho JW, Ikeda S, Polster S, John R, et al. Heart failure-associated changes in RNA splicing of sarcomere genes. Circulation Cardiovascular genetics. 2010;3(2):138–46. doi: 10.1161/CIRCGENETICS.109.904698 - DOI - PMC - PubMed
-
- Weeland CJ, van den Hoogenhof MM, Beqqali A, Creemers EE. Insights into alternative splicing of sarcomeric genes in the heart. Journal of molecular and cellular cardiology. 2015;81:107–13. doi: 10.1016/j.yjmcc.2015.02.008 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

