State Medicaid Hepatitis C Treatment Eligibility Criteria and Use of Direct-Acting Antivirals
- PMID: 29206910
- PMCID: PMC6248367
- DOI: 10.1093/cid/cix1062
State Medicaid Hepatitis C Treatment Eligibility Criteria and Use of Direct-Acting Antivirals
Abstract
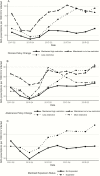
Medicaid program criteria for accessing hepatitis C treatment are changing. Medicaid drug utilization data from 2014 to 2016 show that programs that have relaxed their criteria have seen significant increases in treatment utilization, as have states with Medicaid expansions.
Figures
References
-
- Committee on a National Strategy for the Elimination of Hepatitis B and C. A national strategy for the elimination of hepatitis B and C: phase two report. Washington, DC: The National Academies Press; 2017. - PubMed
-
- Johnson RL, Blumen HE, Ferro C. The burden of hepatitis C virus disease in commercial and managed Medicaid populations Available at: http://us.milliman.com/uploadedFiles/insight/2015/milliman-hcv-burden.pdf. Accessed 7 February 2017.
-
- National Viral Hepatitis Roundtable and the Center for Health Law and Policy Innovation. Hepatitis C: the state of Medicaid access. Washington, DC, 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

