Key Factors Influencing the Emergence of Human Immunodeficiency Virus Drug Resistance in Low- and Middle-Income Countries
- PMID: 29207000
- PMCID: PMC5853971
- DOI: 10.1093/infdis/jix409
Key Factors Influencing the Emergence of Human Immunodeficiency Virus Drug Resistance in Low- and Middle-Income Countries
Abstract
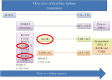
The emergence and spread of human immunodeficiency virus (HIV) drug resistance from antiretroviral roll-out programs remain a threat to long-term control of the HIV-AIDS epidemic in low- and middle-income countries (LMICs). The patterns of drug resistance and factors driving emergence of resistance are complex and multifactorial. The key drivers of drug resistance in LMICs are reviewed here, and recommendations are made to limit their influence on antiretroviral therapy efficacy.
Keywords: HIV subtype; HIV-1 drug resistance; antiretroviral therapy failure.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Reply to Suthar et al.J Infect Dis. 2019 Jan 29;219(4):673-674. doi: 10.1093/infdis/jiy558. J Infect Dis. 2019. PMID: 30307562 Free PMC article. No abstract available.
-
Antimicrobial Resistance and Substandard and Falsified Medicines: The Case of HIV/AIDS.J Infect Dis. 2019 Jan 29;219(4):672-673. doi: 10.1093/infdis/jiy557. J Infect Dis. 2019. PMID: 30307565 Free PMC article. No abstract available.
References
-
- UNAIDS. Global AIDS update http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update.... Accessed 8 July 2017.
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection recommendations for a public health approach, 2nd ed. http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 8 July 2017.
-
- Hamers RL, Wallis CL, Kityo C et al. ; PharmAccess African Studies to Evaluate Resistance (PASER) HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis 2011; 11:750–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

