Hepatocellular carcinoma-targeted nanoparticles for cancer therapy
- PMID: 29207071
- PMCID: PMC5741373
- DOI: 10.3892/ijo.2017.4205
Hepatocellular carcinoma-targeted nanoparticles for cancer therapy
Abstract
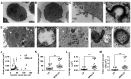
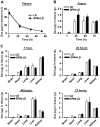
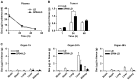
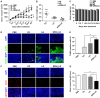
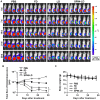
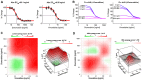
Nanocarriers, such as liposomes, have the potential to increase the payload of chemotherapeutic drugs while decreasing toxicity to non-target tissues; such advantageous properties can be further enhanced through surface conjugation of nanocarriers with targeting moieties. We previously reported that SP94 peptides, identified by phage display, exhibited higher binding affinity to human hepatocellular carcinoma (HCC) than to hepatocytes and other normal cells. Here, we confirm the tumor-targeting properties of SP94 peptide by near-infrared fluorescence imaging. Non-targeted PEGylated liposomal doxorubicin (LD) and SP94‑conjugated PEGylated liposomal doxorubicin (SP94‑LD) were compared by assessing pharmacokinetics, tissue distribution, and antitumor efficacy in xenograft-bearing mice, in order to investigate the effectiveness of SP94‑mediated targeting for cancer therapy. SP94‑LD demonstrated a significant increase in drug accumulation in tumors, while its plasma residence time was the same as its non-targeted equivalent. Consistent with this result, conjugation of targeting peptide SP94 enhances the therapeutic efficacy of liposomal doxorubicin in mouse models with hepatocellular carcinoma xenografts. Furthermore, combination targeted therapy exhibited a significant enhancement against orthotopic tumor growth, and markedly extended the survival of mice compared with all other treatments. Our study shows that SP94‑mediated targeting enhances antitumor efficacy by improving tumor pharmacokinetics and tissue distribution, allowing large amounts of antitumor drugs to accumulate in tumors.
Figures


Similar articles
-
Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer.Biomaterials. 2016 Aug;99:1-15. doi: 10.1016/j.biomaterials.2016.05.015. Epub 2016 May 12. Biomaterials. 2016. PMID: 27209258
-
Lung Cancer-Targeting Peptides with Multi-subtype Indication for Combinational Drug Delivery and Molecular Imaging.Theranostics. 2017 Apr 10;7(6):1612-1632. doi: 10.7150/thno.17573. eCollection 2017. Theranostics. 2017. PMID: 28529640 Free PMC article.
-
A stabilized retro-inverso peptide ligand of transferrin receptor for enhanced liposome-based hepatocellular carcinoma-targeted drug delivery.Acta Biomater. 2019 Jan 1;83:379-389. doi: 10.1016/j.actbio.2018.11.002. Epub 2018 Nov 3. Acta Biomater. 2019. PMID: 30395963
-
Nanoparticles for targeted delivery of therapeutics and small interfering RNAs in hepatocellular carcinoma.World J Gastroenterol. 2015 Nov 14;21(42):12022-41. doi: 10.3748/wjg.v21.i42.12022. World J Gastroenterol. 2015. PMID: 26576089 Free PMC article. Review.
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
Cited by
-
Recent advances of novel targeted drug delivery systems based on natural medicine monomers against hepatocellular carcinoma.Heliyon. 2024 Jan 18;10(2):e24667. doi: 10.1016/j.heliyon.2024.e24667. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38312669 Free PMC article. Review.
-
Sorafenib-Drug Delivery Strategies in Primary Liver Cancer.J Funct Biomater. 2025 Apr 21;16(4):148. doi: 10.3390/jfb16040148. J Funct Biomater. 2025. PMID: 40278256 Free PMC article. Review.
-
Phage Display as a Medium for Target Therapy Based Drug Discovery, Review and Update.Mol Biotechnol. 2025 Jun;67(6):2161-2184. doi: 10.1007/s12033-024-01195-6. Epub 2024 Jun 1. Mol Biotechnol. 2025. PMID: 38822912 Review.
-
Nanomedicine in Hepatocellular Carcinoma: A New Frontier in Targeted Cancer Treatment.Pharmaceutics. 2021 Dec 25;14(1):41. doi: 10.3390/pharmaceutics14010041. Pharmaceutics. 2021. PMID: 35056937 Free PMC article. Review.
-
Extracellular Vesicle-Based Nucleic Acid Delivery: Current Advances and Future Perspectives in Cancer Therapeutic Strategies.Pharmaceutics. 2020 Oct 16;12(10):980. doi: 10.3390/pharmaceutics12100980. Pharmaceutics. 2020. PMID: 33081417 Free PMC article. Review.
References
-
- Souto E. Lipid Nanocarriers in Cancer Diagnosis and Therapy. iSmithers Rapra Publishing; Shrewsbury: 2011.
-
- Lopes de Menezes DE, Pilarski LM, Allen TM. In vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphoma. Cancer Res. 1998;58:3320–3330. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

