Platelet-rich plasma inhibits RANKL-induced osteoclast differentiation through activation of Wnt pathway during bone remodeling
- PMID: 29207140
- PMCID: PMC5752241
- DOI: 10.3892/ijmm.2017.3258
Platelet-rich plasma inhibits RANKL-induced osteoclast differentiation through activation of Wnt pathway during bone remodeling
Abstract
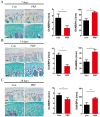
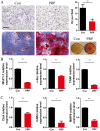
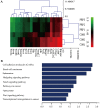
Platelet-rich plasma (PRP) is used in the clinic as an autologous blood product to stimulate bone regeneration and chondrogenesis. Numerous studies have demonstrated that PRP affects bone remodeling by accelerating osteoblast formation. With the research perspective focusing on osteoclasts, the present study established a mouse model of mandibular advancement to examine the effect of PRP on osteoclast differentiation induced by modification of the dynamics of the temporomandibular joint (TMJ). The lower incisors of the mice were trimmed by 1 mm and the resultant change in mandibular position during the process of eating induced condylar adaptation to this change. PRP significantly increased the bone mass and decreased osteoclastic activity, in vitro as well as in vivo. Mechanistically, the reduced expression of receptor activator of nuclear factor-κB ligand (RANKL)‑induced differentiation marker genes, including nuclear factor of activated T-cells, cytoplasmic 1, c-fos and tartrate-resistant acid phosphatase, and that of the resorptive activity marker genes such as cathepsin k, carbonic anhydrase 2 and matrix metalloproteinase 9, indicated that PRP suppresses RANKL-induced osteoclast differentiation. A microarray analysis revealed that several genes associated with the Wnt pathway were differentially expressed, which indicated the involvement of this pathway in osteoclast differentiation. Furthermore, the activation of the Wnt pathway was verified by reverse transcription-quantitative polymerase chain reaction and immunoblot analysis of Dickkopf-related protein 1 and β-catenin. The results of the present study indicated that PRP inhibits osteoclast differentiation through activation of the Wnt pathway.
Figures


Similar articles
-
Ginsenoside Rb2 inhibits osteoclast differentiation through nuclear factor-kappaB and signal transducer and activator of transcription protein 3 signaling pathway.Biomed Pharmacother. 2017 Aug;92:927-934. doi: 10.1016/j.biopha.2017.05.115. Epub 2017 Jun 8. Biomed Pharmacother. 2017. PMID: 28605877
-
The inhibitory effect and the molecular mechanism of glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells.Int J Mol Med. 2012 Feb;29(2):169-77. doi: 10.3892/ijmm.2011.822. Epub 2011 Oct 31. Int J Mol Med. 2012. PMID: 22038020
-
Receptor activator of NF-kappaB ligand induces the expression of carbonic anhydrase II, cathepsin K, and matrix metalloproteinase-9 in osteoclast precursor RAW264.7 cells.Life Sci. 2007 Mar 13;80(14):1311-8. doi: 10.1016/j.lfs.2006.12.037. Epub 2007 Jan 23. Life Sci. 2007. PMID: 17306833
-
Roles of Wnt signals in bone resorption during physiological and pathological states.J Mol Med (Berl). 2013 Jan;91(1):15-23. doi: 10.1007/s00109-012-0974-0. Epub 2012 Oct 31. J Mol Med (Berl). 2013. PMID: 23111637 Review.
-
An update on the role of RANKL-RANK/osteoprotegerin and WNT-ß-catenin signaling pathways in pediatric diseases.World J Pediatr. 2019 Feb;15(1):4-11. doi: 10.1007/s12519-018-0198-7. Epub 2018 Oct 20. World J Pediatr. 2019. PMID: 30343446
Cited by
-
Progress and Applications of Polyphosphate in Bone and Cartilage Regeneration.Biomed Res Int. 2019 Jun 27;2019:5141204. doi: 10.1155/2019/5141204. eCollection 2019. Biomed Res Int. 2019. PMID: 31346519 Free PMC article. Review.
-
Platelet-Rich Plasma in Treatment of Temporomandibular Joint Dysfunctions: Narrative Review.Int J Mol Sci. 2019 Jan 11;20(2):277. doi: 10.3390/ijms20020277. Int J Mol Sci. 2019. PMID: 30641957 Free PMC article. Review.
-
In Situ Casting of Platelet Rich Plasma/SiO2/Alginate for Bone Tissue Engineering Application in Rabbit Mandible Defect Model.J Dent (Shiraz). 2022 Sep;23(2 Suppl):349-360. doi: 10.30476/DENTJODS.2021.90677.1513. J Dent (Shiraz). 2022. PMID: 36588966 Free PMC article.
-
[Effectiveness and mechanism of pure platelet-rich plasma on osteochondral injury of talus].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019 May 15;33(5):555-562. doi: 10.7507/1002-1892.201811096. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019. PMID: 31090348 Free PMC article. Clinical Trial. Chinese.
-
Local Wnt3a treatment restores bone regeneration in large osseous defects after surgical debridement of osteomyelitis.J Mol Med (Berl). 2020 Jun;98(6):897-906. doi: 10.1007/s00109-020-01924-9. Epub 2020 May 18. J Mol Med (Berl). 2020. PMID: 32424558 Free PMC article.
References
-
- Ruf S, Pancherz H. Temporomandibular joint remodeling in adolescents and young adults during Herbst treatment: A prospective longitudinal magnetic resonance imaging and cephalometric radiographic investigation. Am J Orthod Dentofacial Orthop. 1999;115:607–618. doi: 10.1016/S0889-5406(99)70285-4. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

