miR-33a hinders the differentiation of adipose mesenchymal stem cells towards urothelial cells in an inductive condition by targeting β‑catenin and TGFR
- PMID: 29207162
- PMCID: PMC5783476
- DOI: 10.3892/mmr.2017.8168
miR-33a hinders the differentiation of adipose mesenchymal stem cells towards urothelial cells in an inductive condition by targeting β‑catenin and TGFR
Abstract
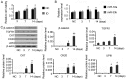
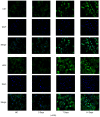
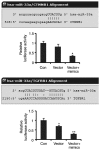
Tissue engineering technology offers an appealing approach for tissue reconstruction of the urothelium. Adipose‑derived mesenchymal stem cells (ADSCs) represent an abundant source for tissue engineering applications. However, ASCs primarily possess mesoderm lineage differentiation potential. It is difficult to induce differentiation of ASCs towards urothelial cells that are derived from the endoderm, although a recent findings have reported that a conditioned medium may drive ADSCs towards differentiation into the urothelium phenotype. In the present study, human ADSCs were isolated from abdominal adipose tissues and incubated in this conditioned medium for indicated time periods. Western blotting showed that protein expression levels of urothelial specific marks, including CK7, CK20 and UPIII, were increased after seven days' incubation, but immunofluorescence microscopy determined that cells with CK7 and UPIII staining were scarce, which suggested a low‑efficiency for the differentiation. Prolonging the incubation time did not further increase CK20 and UPIII expression. Furthermore, miR‑33a expression was increased with ADSC differentiation. Using synthetic miRNAs to mimic or inhibit the action of miR‑33a revealed that miR‑33a hinders the differentiation of ADSCs towards urothelial cells. Furthermore, luciferase reporter assay confirmed that β‑catenin and transforming growth factor‑β receptor (TGFR) are targets of miR‑33a. Inhibition of miR‑33a expression increased β‑catenin and TGFR expression and improved the efficiency of ADSCs towards differentiation into the urothelium phenotype. The present novel finding suggests that miR‑33 may be an important target in tissue engineering and regenerative medicine for urothelium repair.
Figures



Similar articles
-
Adipose-derived stem cells undergo differentiation after co-culture with porcine limbal epithelial stem cells.Stem Cell Res. 2019 Dec;41:101609. doi: 10.1016/j.scr.2019.101609. Epub 2019 Oct 24. Stem Cell Res. 2019. PMID: 31706096
-
Differentiation of human adipose-derived stem cells co-cultured with urothelium cell line toward a urothelium-like phenotype in a nude murine model.Urology. 2013 Feb;81(2):465.e15-22. doi: 10.1016/j.urology.2012.10.030. Urology. 2013. PMID: 23374843
-
[Wnt/β-catenin pathway involved in the regulation of rat mesangial cell proliferation by adipose-derived mesenchymal stem cells].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016 Nov;32(11):1486-1490. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016. PMID: 27774940 Chinese.
-
The role of epigenetic modifications in the osteogenic differentiation of adipose-derived stem cells.Connect Tissue Res. 2019 Nov;60(6):507-520. doi: 10.1080/03008207.2019.1593395. Epub 2019 Jun 17. Connect Tissue Res. 2019. PMID: 31203665 Review.
-
Bone Marrow- and Adipose Tissue-Derived Mesenchymal Stem Cells: Characterization, Differentiation, and Applications in Cartilage Tissue Engineering.Crit Rev Eukaryot Gene Expr. 2018;28(4):285-310. doi: 10.1615/CritRevEukaryotGeneExpr.2018023572. Crit Rev Eukaryot Gene Expr. 2018. PMID: 30311578 Review.
Cited by
-
Construction of lncRNA- and circRNA-associated ceRNA networks in the prostatic urethra of rats after simulating transurethral laser prostatectomy (TULP).Mol Cell Biochem. 2024 Jun;479(6):1363-1377. doi: 10.1007/s11010-023-04804-1. Epub 2023 Jul 6. Mol Cell Biochem. 2024. PMID: 37410211 Free PMC article.
-
miR-33a Inhibits the Differentiation of Bovine Preadipocytes through the IRS2-Akt Pathway.Genes (Basel). 2023 Feb 20;14(2):529. doi: 10.3390/genes14020529. Genes (Basel). 2023. PMID: 36833456 Free PMC article.
References
-
- Tanthaisong P, Imsoonthornruksa S, Ngernsoungnern A, Ngernsoungnern P, Ketudat-Cairns M, Parnpai R. Enhanced chondrogenic differentiation of human umbilical cord wharton's jelly derived mesenchymal stem cells by GSK-3 inhibitors. PLoS One. 2017;12:e0168059. doi: 10.1371/journal.pone.0168059. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

