Pentosan polysulfate ameliorates apoptosis and inflammation by suppressing activation of the p38 MAPK pathway in high glucose‑treated HK‑2 cells
- PMID: 29207166
- PMCID: PMC5752165
- DOI: 10.3892/ijmm.2017.3290
Pentosan polysulfate ameliorates apoptosis and inflammation by suppressing activation of the p38 MAPK pathway in high glucose‑treated HK‑2 cells
Abstract
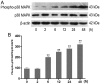
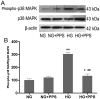
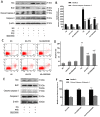
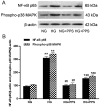
The apoptosis of tubular epithelial cells in diabetic nephropathy (DN) is commonly observed in human renal biopsies. Inflammation plays a key role in DN, and pentosan polysulfate (PPS) has been shown to largely attenuate the inflammation of nephropathy in aging diabetic mice. p38 mitogen‑activated protein kinase (p38 MAPK) plays a crucial role in tissue inflammation and cell apoptosis, and it is activated by hyperglycemia. In the present study, high glucose (HG)‑treated human renal proximal tubular epithelial cells (HK‑2) were used to examine the protective effects of PPS against HG‑stimulated apoptosis and inflammation. The results of the study revealed that PPS markedly suppressed the HG‑induced reduction in cell viability. Incubation of HK‑2 cells with HG activated the p38 MAPK pathway and, subsequently, as confirmed by western blot analysis and flow cytometry, increased cell apoptosis, which was blocked by PPS. In addition, PPS treatment significantly inhibited HG‑stimulated p38 MAPK and nuclear factor‑κB activation, and reduced the production of pro‑inflammatory cytokines, such as tumor necrosis factor‑α, interleukin (IL)‑1β and IL‑6. In conclusion, PPS ameliorates p38 MAPK‑mediated renal cell apoptosis and inflammation. The anti‑apoptotic actions and anti‑inflammatory effects of PPS prompt further investigation of this compound as a promising therapeutic agent against DN.
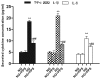
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

