Neuroimaging in Dementia
- PMID: 29207412
- PMCID: PMC5823524
- DOI: 10.1055/s-0037-1608808
Neuroimaging in Dementia
Abstract
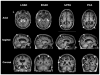
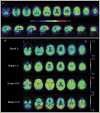
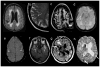
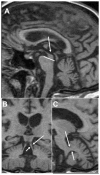
Although the diagnosis of dementia still is primarily based on clinical criteria, neuroimaging is playing an increasingly important role. This is in large part due to advances in techniques that can assist with discriminating between different syndromes. Magnetic resonance imaging remains at the core of differential diagnosis, with specific patterns of cortical and subcortical changes having diagnostic significance. Recent developments in molecular PET imaging techniques have opened the door for not only antemortem but early, even preclinical, diagnosis of underlying pathology. This is vital, as treatment trials are underway for pharmacological agents with specific molecular targets, and numerous failed trials suggest that earlier treatment is needed. This article provides an overview of classic neuroimaging findings as well as new and cutting-edge research techniques that assist with clinical diagnosis of a range of dementia syndromes, with an emphasis on studies using pathologically proven cases.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

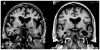
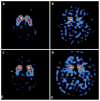
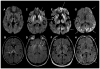
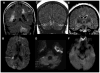
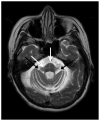
References
-
- Crutch SJ, Schott JM, Rabinovici GD, et al. Alzheimer’s Association ISTAART Atypical Alzheimer’s Disease and Associated Syndromes Professional Interest Area. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13(08):870–884. doi: 10.1016/j.jalz.2017.01.014. - DOI - PMC - PubMed
-
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(03):263–269. doi: 10.1016/j.jalz.2011.03.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

