Changes in renal function indices in cirrhotic chronic hepatitis C patients treated with sofosbuvir-containing regimens
- PMID: 29207613
- PMCID: PMC5710894
- DOI: 10.18632/oncotarget.18701
Changes in renal function indices in cirrhotic chronic hepatitis C patients treated with sofosbuvir-containing regimens
Abstract
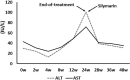
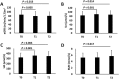
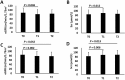
This study aimed to explore changes in hepatic and renal function indices in chronic hepatitis C (CHC) patients treated with direct-acting antivirals (DAAs). Forty-three CHC patients treated with sofosbuvir (SOF)-containing regimens were enrolled. At the end of treatment, the estimated glomerular filtration rate (eGFR) level was significantly decreased and the serum creatinine (Scr) and uric acid (UA) levels were significantly increased compared with baseline levels (eGFR: 86.7 ± 20.4 vs 80.5 ± 21.3, P01 = 0.005; Scr: 83.9 ± 19.1 vs 89.6 ± 21.1, P01 < 0.001; UA: 323.7± 86.2 vs 358.5 ± 93.2, P01 < 0.001); no significant improvements were observed at 24 w post-treatment (eGFR: 86.7 ± 20.4 vs 81.4 ± 18.6, P02 = 0.013; Scr: 83.6 ± 17.9 vs 87.9 ± 18.3, P02 = 0.014; UA: 320.8 ± 76.3 vs 349.3 ± 91.0, P02 = 0.004). When the patients were grouped by liver conditions, non-cirrhotic patients and cirrhotic patients had decreased eGFR levels and increased Scr levels at the end of treatment; at 24 w post-treatment, the eGFR and Scr levels were significantly improved in non-cirrhotic patients (88.4 ± 21.7 vs 83.8 ± 18.5, P02 = 0.142; 84.4 ± 20.4 vs 87.0 ± 16.9, P02 = 0.088), while no obvious improvements were observed in cirrhotic patients (84.3 ± 18.7 vs 78.1 ± 18.6, P02 = 0.002; 83.2 ± 17.7 vs 89.2 ± 20.6, P02 = 0.006). Clinical physicians should closely monitor renal function in patients treated with SOF-containing regimens, especially in cirrhotic patients.
Keywords: chronic hepatitis C; directly acting antivirals; nephrotoxicity; sofosbuvir.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that no conflicts of interest exists.
Figures
Similar articles
-
Dual treatment with sofosbuvir plus ribavirin is as effective as triple therapy with pegylated interferon plus sofosbuvir plus ribavirin in predominant genotype 3 patients with chronic hepatitis C.J Gastroenterol Hepatol. 2017 Apr;32(4):859-863. doi: 10.1111/jgh.13595. J Gastroenterol Hepatol. 2017. PMID: 27624314
-
Twelve-week ribavirin-free direct-acting antivirals for treatment-experienced Chinese with HCV genotype 1b infection including cirrhotic patients.Hepatol Int. 2016 Sep;10(5):789-98. doi: 10.1007/s12072-016-9755-0. Epub 2016 Jul 21. Hepatol Int. 2016. PMID: 27443347
-
Treatment of hepatitis C virus recurrence after transplantation with sofosbuvir/ledipasvir: The role of ribavirin.Transpl Infect Dis. 2017 Feb;19(1). doi: 10.1111/tid.12647. Epub 2017 Jan 11. Transpl Infect Dis. 2017. PMID: 27943544
-
Pharmacokinetics, Efficacy, and Safety of Hepatitis C Virus Drugs in Patients with Liver and/or Renal Impairment.Drug Saf. 2016 Jul;39(7):589-611. doi: 10.1007/s40264-016-0420-2. Drug Saf. 2016. PMID: 27098247 Free PMC article. Review.
-
Treatment of hepatitis C virus genotype 3 infection with direct-acting antiviral agents.Braz J Med Biol Res. 2016 Oct 24;49(11):e5504. doi: 10.1590/1414-431X20165504. Braz J Med Biol Res. 2016. PMID: 27783808 Free PMC article. Review.
References
-
- Everson GT, Sims KD, Rodriguez-Torres M, Hézode C, Lawitz E, Bourlière M, Loustaud-Ratti V, Rustgi V, Schwartz H, Tatum H, Marcellin P, Pol S, Thuluvath PJ, et al. Efficacy of an interferon- and ribavirin-free regimen of daclatasvir, asunaprevir, and BMS-791325 in treatment-naive patients with HCV genotype 1 infection. Gastroenterology. 2014;146:420–9. doi: 10.1053/j.gastro.2013.10.057. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

