Wheat germ agglutinin-induced paraptosis-like cell death and protective autophagy is mediated by autophagy-linked FYVE inhibition
- PMID: 29207637
- PMCID: PMC5710917
- DOI: 10.18632/oncotarget.20436
Wheat germ agglutinin-induced paraptosis-like cell death and protective autophagy is mediated by autophagy-linked FYVE inhibition
Abstract
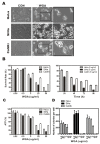
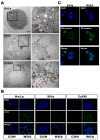
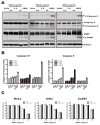
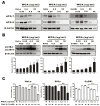
Wheat germ agglutinin (WGA) is a lectin that specifically binds cell surface glycoproteins and disrupts nuclear pore complex function through its interaction with POM121. Our data indicate WGA induces paraptosis-like cell death without caspase activation. We observed the main features of paraptosis, including cytoplasmic vacuolation, endoplasmic reticulum dilation and increased ER stress, and the unfolded protein response in WGA-treated cervical carcinoma cells. Conversion of microtubule-associated protein I light chain 3 (LC3-I) into LC3-II and punctuate formation suggestive of autophagy were observed in WGA-treated cells. WGA-induced autophagy antagonized paraptosis in HeLa and CaSKi cells, which expressed autophagy-linked FYVE (Alfy) protein, but not in SiHa cells that did not express Alfy. Alfy knockdown in HeLa cells induced paraptosis-like cell death. These data indicate that WGA-induced cell death occurs through paraptosis and that autophagy may exert a protective effect. WGA treatment and Alfy inhibition could be an effective therapeutic strategy for apoptosis-resistant cervical cancer cells.
Keywords: Alfy; cell death; cytoplasmic vacuolation; paraptosis; wheat germ agglutinin.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
8-p-Hdroxybenzoyl Tovarol Induces Paraptosis Like Cell Death and Protective Autophagy in Human Cervical Cancer HeLa Cells.Int J Mol Sci. 2015 Jul 2;16(7):14979-96. doi: 10.3390/ijms160714979. Int J Mol Sci. 2015. PMID: 26147427 Free PMC article.
-
Endoplasmic reticulum vacuolation and unfolded protein response leading to paraptosis like cell death in cyclosporine A treated cancer cervix cells is mediated by cyclophilin B inhibition.Biochim Biophys Acta. 2014 Nov;1843(11):2497-512. doi: 10.1016/j.bbamcr.2014.06.020. Epub 2014 Jul 5. Biochim Biophys Acta. 2014. PMID: 25003316
-
Paraptosis accompanied by autophagy and apoptosis was induced by celastrol, a natural compound with influence on proteasome, ER stress and Hsp90.J Cell Physiol. 2012 May;227(5):2196-206. doi: 10.1002/jcp.22956. J Cell Physiol. 2012. PMID: 21866552
-
Small-molecule compounds target paraptosis to improve cancer therapy.Biomed Pharmacother. 2019 Oct;118:109203. doi: 10.1016/j.biopha.2019.109203. Epub 2019 Jul 12. Biomed Pharmacother. 2019. PMID: 31306970 Review.
-
Paraptosis in the anti-cancer arsenal of natural products.Pharmacol Ther. 2016 Jun;162:120-33. doi: 10.1016/j.pharmthera.2016.01.003. Epub 2016 Jan 21. Pharmacol Ther. 2016. PMID: 26802901 Review.
Cited by
-
Glycosylation-Dependent Induction of Programmed Cell Death in Murine Adenocarcinoma Cells.Front Immunol. 2022 Feb 10;13:797759. doi: 10.3389/fimmu.2022.797759. eCollection 2022. Front Immunol. 2022. PMID: 35222379 Free PMC article.
-
Paraptosis: a unique cell death mode for targeting cancer.Front Pharmacol. 2023 Jun 15;14:1159409. doi: 10.3389/fphar.2023.1159409. eCollection 2023. Front Pharmacol. 2023. PMID: 37397502 Free PMC article. Review.
-
Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1.Autophagy. 2021 Jan;17(1):1-382. doi: 10.1080/15548627.2020.1797280. Epub 2021 Feb 8. Autophagy. 2021. PMID: 33634751 Free PMC article.
-
Nuclear pore protein POM121 regulates subcellular localization and transcriptional activity of PPARγ.Cell Death Dis. 2024 Jan 4;15(1):7. doi: 10.1038/s41419-023-06371-1. Cell Death Dis. 2024. PMID: 38177114 Free PMC article.
-
Wheat Germ Agglutinin as a Potential Therapeutic Agent for Leukemia.Front Oncol. 2019 Feb 21;9:100. doi: 10.3389/fonc.2019.00100. eCollection 2019. Front Oncol. 2019. PMID: 30847305 Free PMC article.
References
-
- Li XT, Ju RJ, Li XY, Zeng F, Shi JF, Liu L, Zhang CX, Sun MG, Lou JN, Lu WL. Multifunctional targeting daunorubicin plus quinacrine liposomes, modified by wheat germ agglutinin and tamoxifen, for treating brain glioma and glioma stem cells. Oncotarget. 2014;5:6497–6511. doi: 10.18632/oncotarget.2267. - DOI - PMC - PubMed
-
- Meng Y, Hou X, Lei J, Chen M, Cong S, Zhang Y, King W, Li G, Li X. Multi-function liposomes enhancing target and antibacterial immunity for antimicrobial and anti-biofilm against methicillin-resistant staphylococcus aureus. Pharm Res. 2016;33:763–75. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

