Recurrent glioma clinical trial, CheckMate-143: the game is not over yet
- PMID: 29207684
- PMCID: PMC5710964
- DOI: 10.18632/oncotarget.21586
Recurrent glioma clinical trial, CheckMate-143: the game is not over yet
Abstract
Glioblastoma (GBM) is the most common, and aggressive, primary brain tumor in adults. With a median patient survival of less than two years, GBM represents one of the biggest therapeutic challenges of the modern era. Even with the best available treatment, recurrence rates are nearly 100% and therapeutic options at the time of relapse are extremely limited. Nivolumab, an anti-programmed cell death-1 (PD-1) monoclonal antibody, has provided significant clinical benefits in the treatment of various advanced cancers and represented a promising therapy for primary and recurrent GBM. CheckMate 143 (NCT 02017717) was the first large randomized clinical trial of PD pathway inhibition in the setting of GBM, including a comparison of nivolumab and the anti-VEGF antibody, bevacizumab, in the treatment of recurrent disease. However, preliminary results, recently announced in a WFNOS 2017 abstract, demonstrated a failure of nivolumab to prolong overall survival of patients with recurrent GBM, and this arm of the trial was prematurely closed. In this review, we discuss the basic concepts underlying the rational to target PD pathway in GBM, address implications of using immune checkpoint inhibitors in central nervous system malignancies, provide a rationale for possible reasons contributing to the failure of nivolumab to prolong survival in patients with recurrent disease, and analyze the future role of immune checkpoint inhibitors in the treatment of GBM.
Keywords: PD-1/PD-L1; checkpoint inhibitor; gliolastoma; immunotherapy; malignant glioma.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they have no competing interests.
Figures
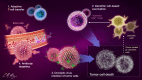
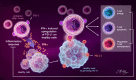
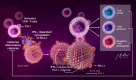
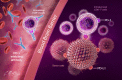
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. - PubMed
-
- Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev. 2012;64:640–65. - PubMed
-
- Miura Y, Takenaka T, Toh K, Wu S, Nishihara H, Kano MR, Ino Y, Nomoto T, Matsumoto Y, Koyama H, Cabral H, Nishiyama N, Kataoka K. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano. 2013;7:8583–92. - PubMed
-
- Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216–32. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

