Diagnosis and Management of Upper Gastrointestinal Neuroendocrine Tumors
- PMID: 29207862
- PMCID: PMC5719910
- DOI: 10.5946/ce.2017.181
Diagnosis and Management of Upper Gastrointestinal Neuroendocrine Tumors
Abstract
Upper gastrointestinal neuroendocrine tumors (NETs) are rare tumors which are increasingly recognised by practising endoscopists. After confirmation by endoscopic biopsies of these focal lesions, many questions may arise. As NETs are less frequently encountered compared to other malignancies or gastrointestinal pathology, many endoscopists may not fully understand the natural history, diagnosis and management of these tumors. In this review, we aim to update the practising endoscopist on the key clinical features and management of patients with upper gastrointestinal NET.
Keywords: Duodenal; Esophageal; Gastric; Neuroendocrine tumor.
Conflict of interest statement
Figures
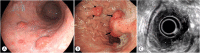
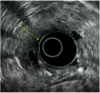
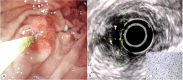
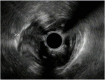
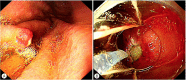
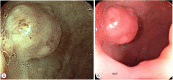
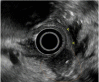
Similar articles
-
Neuroendocrine Tumors of the Gastrointestinal Tract: A Focused Review and Practical Approach for Gastroenterologists.GE Port J Gastroenterol. 2021 Sep;28(5):336-348. doi: 10.1159/000512089. Epub 2021 Jan 20. GE Port J Gastroenterol. 2021. PMID: 34604465 Free PMC article. Review.
-
Evolving role of the endoscopist in management of gastrointestinal neuroendocrine tumors.World J Gastroenterol. 2017 Jul 21;23(27):4847-4855. doi: 10.3748/wjg.v23.i27.4847. World J Gastroenterol. 2017. PMID: 28785139 Free PMC article.
-
Management of gastric and duodenal neuroendocrine tumors.World J Gastroenterol. 2016 Aug 14;22(30):6817-28. doi: 10.3748/wjg.v22.i30.6817. World J Gastroenterol. 2016. PMID: 27570419 Free PMC article. Review.
-
AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States.Clin Gastroenterol Hepatol. 2019 Jan;17(1):16-25.e1. doi: 10.1016/j.cgh.2018.07.041. Epub 2018 Aug 2. Clin Gastroenterol Hepatol. 2019. PMID: 30077787 Review.
-
Efficacy of endoscopic ultrasound-guided fine-needle aspiration and core needle biopsy in the diagnosis of upper gastrointestinal submucosal lesions.J Am Soc Cytopathol. 2017 Nov-Dec;6(6):254-264. doi: 10.1016/j.jasc.2017.07.003. Epub 2017 Jul 27. J Am Soc Cytopathol. 2017. PMID: 31043296
Cited by
-
Last decade of advances in gastric neuroendocrine tumors: Innovations, challenges, and future directions.World J Clin Oncol. 2025 May 24;16(5):104577. doi: 10.5306/wjco.v16.i5.104577. World J Clin Oncol. 2025. PMID: 40503415 Free PMC article.
-
Neuroendocrine neoplasm of the gallbladder: Clinical features, surgical efficacy, and prognosis.Cancer Med. 2023 May;12(10):11344-11350. doi: 10.1002/cam4.5846. Epub 2023 Mar 23. Cancer Med. 2023. PMID: 36952352 Free PMC article.
-
Neuroendocrine Carcinoma of Duodenum-an Uncommon Tumour at an Unusual Site.Indian J Surg Oncol. 2019 Mar;10(1):199-203. doi: 10.1007/s13193-018-0834-7. Epub 2018 Dec 7. Indian J Surg Oncol. 2019. PMID: 30948899 Free PMC article.
-
Current updates and future directions in diagnosis and management of gastroenteropancreatic neuroendocrine neoplasms.World J Gastrointest Endosc. 2022 May 16;14(5):267-290. doi: 10.4253/wjge.v14.i5.267. World J Gastrointest Endosc. 2022. PMID: 35719897 Free PMC article. Review.
-
Successful endoscopic resection of an unusually enlarged and pedunculated type I gastric carcinoid tumour.BMJ Case Rep. 2021 Aug 19;14(8):e244292. doi: 10.1136/bcr-2021-244292. BMJ Case Rep. 2021. PMID: 34413045 Free PMC article.
References
-
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. - PubMed
-
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. - PubMed
-
- Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol. 2004;99:23–32. - PubMed
-
- Huang Q, Wu H, Nie L, et al. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013;37:467–483. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

