Decreased expression of the β2 integrin on tumor cells is associated with a reduction in liver metastasis of colorectal cancer in mice
- PMID: 29207960
- PMCID: PMC5718006
- DOI: 10.1186/s12885-017-3823-2
Decreased expression of the β2 integrin on tumor cells is associated with a reduction in liver metastasis of colorectal cancer in mice
Abstract
Background: Lymphocyte Function-Associated Antigen-1 (LFA-1; CD18/CD11a) is one of the main adhesion molecules used by immune cells to infiltrate the liver under inflammatory conditions. Recently, the expression of this integrin has also been reported on several solid tumors, including colorectal cancer. However, its functional role in the metastatic progression to the liver remains unknown. Using in vitro assays and an experimental orthotopic in vivo model of liver metastasis, we aimed to elucidate the role of tumor LFA-1 in the metastatic progression by means of the partial depletion of the β2 subunit of LFA-1, required for integrin activation, firm adhesion and signaling.
Methods: To do so, we evaluated the effects of β2 reduction on the murine colon carcinoma C26 cell line on their pro-metastatic features in vitro and their metastatic potential in vivo in a mouse model of colon carcinoma metastasis to the liver.
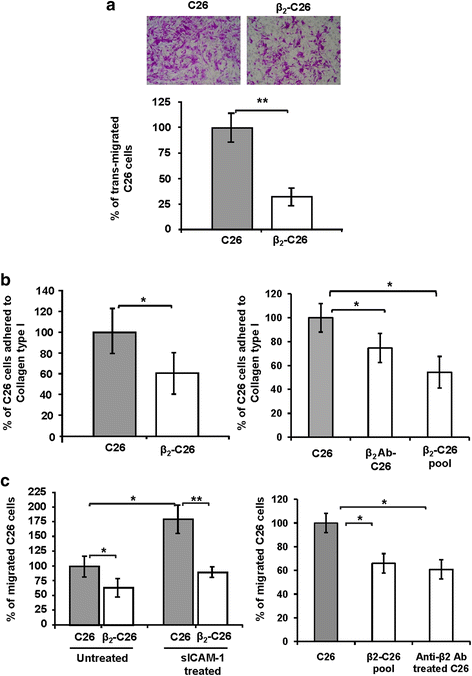
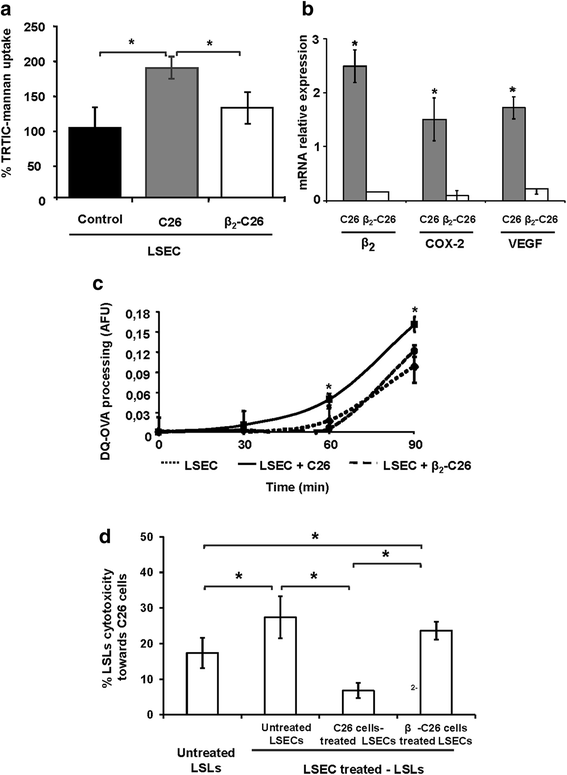
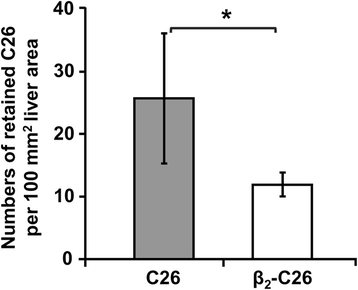
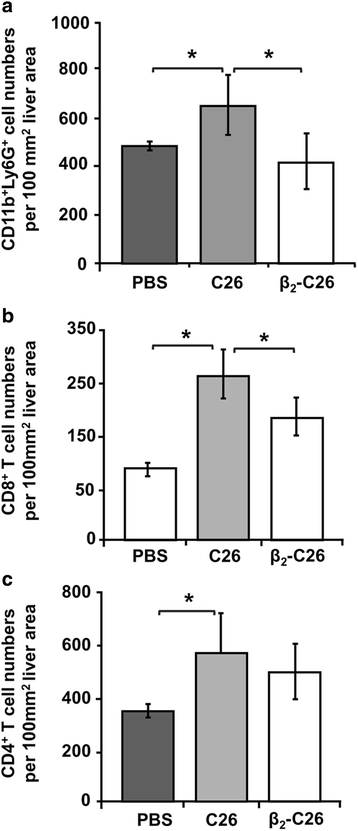
Results: The reduction in β2 integrin expression correlated with a slower proliferation, and a reduced adhesion and migration of C26 cells in an in vitro setting. Additionally, tumor cells with a reduced in β2 integrin expression were unable to activate the liver sinusoidal endothelial cells (LSECs). This resulted in a recovery of the cytotoxic potential of liver lymphocytes which is compromised by LSECs activated by C26 cells. This was related to the abrogation of RNA expression of inflammatory and angiogenic cytokines by C26 cells after their activation with sICAM-1, the main ligand of β2αL. Furthermore, in vivo tumor cell retention and metastasis were profoundly reduced, along with a decrease in the recruitment and infiltration of myeloid derived suppressor cells (MDSCs) and lymphocytes to the liver.
Conclusion: Taken together, our findings uncovered the modulatory role for the tumor β2 subunit of the LFA-1 integrin in the metastatic progression of colorectal cancer to the liver by impairing activation of liver endothelium and thus, the local immune response in the liver. Besides, this integrin also showed to be critical in vivo for tumor cell retention, cytokine release, leukocyte recruitment and metastasis development. These data support a therapeutical potential of the integrin LFA-1 as a target for the treatment of colorectal liver metastasis.
Keywords: Colorectal cancer; Endothelial cells; ICAM-1; Immune response; LFA-1; Liver metastasis; Tumor microenvironment; β2 integrin.
Conflict of interest statement
Ethics approval
All the animal proceedings were approved by the Basque Country University Ethical Committee (CEID) in accordance with institutional, national and international guidelines regarding the protection and care of animals use for scientific purposes.
Consent for publication
No applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





Similar articles
-
Liver sinusoidal endothelial cell ICAM-1 mediated tumor/endothelial crosstalk drives the development of liver metastasis by initiating inflammatory and angiogenic responses.Sci Rep. 2019 Sep 11;9(1):13111. doi: 10.1038/s41598-019-49473-7. Sci Rep. 2019. PMID: 31511625 Free PMC article.
-
Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma.J Immunol. 1998 Apr 15;160(8):3978-88. J Immunol. 1998. PMID: 9558106
-
αL β2 integrin is indispensable for CD8+ T-cell recruitment in experimental pancreatic and hepatocellular cancer.Int J Cancer. 2012 May 1;130(9):2067-76. doi: 10.1002/ijc.26223. Epub 2011 Aug 16. Int J Cancer. 2012. PMID: 21647874
-
The Role of LFA-1 for the Differentiation and Function of Regulatory T Cells-Lessons Learned from Different Transgenic Mouse Models.Int J Mol Sci. 2023 Mar 28;24(7):6331. doi: 10.3390/ijms24076331. Int J Mol Sci. 2023. PMID: 37047302 Free PMC article. Review.
-
β2 Integrins-Multi-Functional Leukocyte Receptors in Health and Disease.Int J Mol Sci. 2020 Feb 19;21(4):1402. doi: 10.3390/ijms21041402. Int J Mol Sci. 2020. PMID: 32092981 Free PMC article. Review.
Cited by
-
Impact of Fibrinogen, Fibrin Thrombi, and Thrombin on Cancer Cell Extravasation Using In Vitro Microvascular Networks.Adv Healthc Mater. 2023 Jul;12(19):e2202984. doi: 10.1002/adhm.202202984. Epub 2023 May 12. Adv Healthc Mater. 2023. PMID: 37119127 Free PMC article.
-
Extracellular Collagen Mediates Osteosarcoma Progression Through an Integrin α2β1/JAK/STAT3 Signaling Pathway.Cancer Manag Res. 2020 Nov 24;12:12067-12075. doi: 10.2147/CMAR.S273466. eCollection 2020. Cancer Manag Res. 2020. PMID: 33262655 Free PMC article.
-
Neutrophils in cancer carcinogenesis and metastasis.J Hematol Oncol. 2021 Oct 21;14(1):173. doi: 10.1186/s13045-021-01187-y. J Hematol Oncol. 2021. PMID: 34674757 Free PMC article. Review.
-
Development of a Microphysiological System to Model Human Cancer Metastasis From the Colon to the Liver.Biotechnol Bioeng. 2025 Mar;122(3):481-494. doi: 10.1002/bit.28890. Epub 2024 Nov 25. Biotechnol Bioeng. 2025. PMID: 39587032
-
Liver Immune Microenvironment and Metastasis from Colorectal Cancer-Pathogenesis and Therapeutic Perspectives.Cancers (Basel). 2021 May 17;13(10):2418. doi: 10.3390/cancers13102418. Cancers (Basel). 2021. PMID: 34067719 Free PMC article. Review.
References
-
- van Grevenstein WM, Hofland LJ, Jeekel J, van Eijck CH. The expression of adhesion molecules and the influence of inflammatory cytokines on the adhesion of human pancreatic carcinoma cells to mesothelial monolayers. Pancreas. 2006;32(4):396–402. doi: 10.1097/01.mpa.0000220865.80034.2a. - DOI - PubMed
-
- Arteta B, Lasuen N, Lopategi A, Sveinbjörnsson B, Smedsrød B, Vidal-Vanaclocha F. Colon carcinoma cell interaction with liver sinusoidal endothelium inhibits organ-specific antitumor immunity through interleukin-1-induced mannose receptor in mice. Hepatology. 2010;51(6):2172–2182. doi: 10.1002/hep.23590. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

