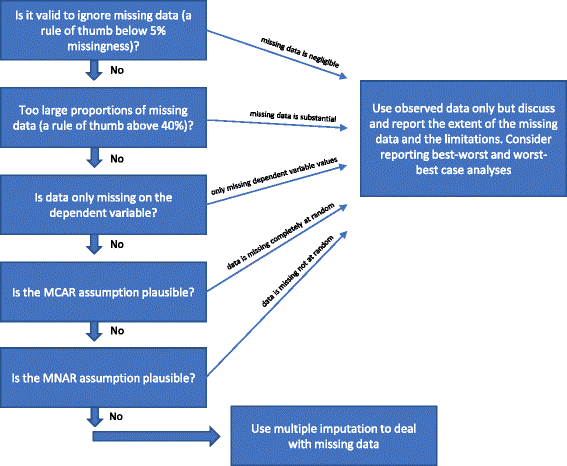
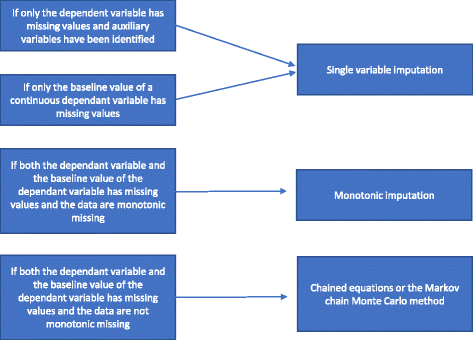
When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts
- PMID: 29207961
- PMCID: PMC5717805
- DOI: 10.1186/s12874-017-0442-1
When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts
Abstract
Background: Missing data may seriously compromise inferences from randomised clinical trials, especially if missing data are not handled appropriately. The potential bias due to missing data depends on the mechanism causing the data to be missing, and the analytical methods applied to amend the missingness. Therefore, the analysis of trial data with missing values requires careful planning and attention.
Methods: The authors had several meetings and discussions considering optimal ways of handling missing data to minimise the bias potential. We also searched PubMed (key words: missing data; randomi*; statistical analysis) and reference lists of known studies for papers (theoretical papers; empirical studies; simulation studies; etc.) on how to deal with missing data when analysing randomised clinical trials.
Results: Handling missing data is an important, yet difficult and complex task when analysing results of randomised clinical trials. We consider how to optimise the handling of missing data during the planning stage of a randomised clinical trial and recommend analytical approaches which may prevent bias caused by unavoidable missing data. We consider the strengths and limitations of using of best-worst and worst-best sensitivity analyses, multiple imputation, and full information maximum likelihood. We also present practical flowcharts on how to deal with missing data and an overview of the steps that always need to be considered during the analysis stage of a trial.
Conclusions: We present a practical guide and flowcharts describing when and how multiple imputation should be used to handle missing data in randomised clinical.
Keywords: Missing data; Multiple imputation; Randomised clinical trials.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical