Study protocol for a cluster-randomized trial to compare human papillomavirus based cervical cancer screening in community-health campaigns versus health facilities in western Kenya
- PMID: 29207966
- PMCID: PMC5717798
- DOI: 10.1186/s12885-017-3818-z
Study protocol for a cluster-randomized trial to compare human papillomavirus based cervical cancer screening in community-health campaigns versus health facilities in western Kenya
Abstract
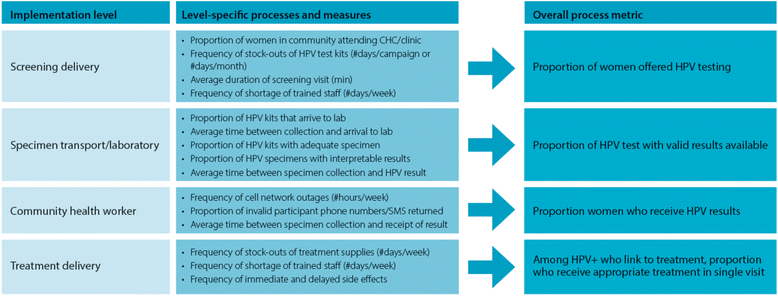
Background: Despite guidelines for cervical cancer prevention in low-resource countries, a very small proportion of women in these settings undergo screening, and even fewer women are successfully treated. Using pilot data from western Kenya and World Health Organization recommendations, we developed a protocol to implement evidence-based cervical cancer screening and linkage to treatment strategies to the rural communities. We describe the protocol for a cluster-randomized trial to compare two implementation strategies for human-papillomavirus (HPV)-based cervical cancer screening program using metrics described in the RE-AIM (reach, efficacy, adaption, implementation and maintenance) framework.
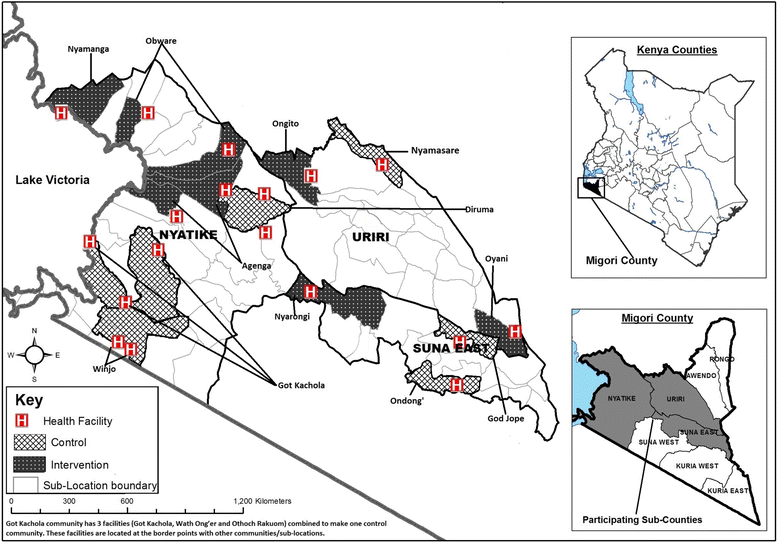
Methods: The study is a three-year, two-phase cluster-randomized trial in 18 communities in western Kenya. During Phase 1, six control communities were offered screening in health facilities; and six intervention communities were offered screening in community health campaigns. Screening was done with human-papillomavirus testing through self-collected specimens. Phase 1 ended and we are working in partnership with communities to further contextualize the implementation strategy for screening, and develop an enhanced linkage to treatment plan. This plan will be tested in an additional six communities in Phase 2 (enhanced intervention). We will compare the reach, efficacy, cost-effectiveness and adaptability of the implementation strategies.
Discussion: Effective low-cost cervical cancer prevention technologies are becoming more widely available in low- and middle-income countries. Despite increasing government support for cervical cancer prevention, there remains a sizeable gap in service availability. We will use implementation science to identify the most effective strategies to fill this gap through development of context-specific evidence-based solutions. This protocol design and results can help guide implementation of cervical cancer screening in similar settings, where women are most underserved and at highest risk for disease.
Trial registration: This trial is registered at ClinicalTrials.gov , NCT02124252 .
Keywords: Cervical cancer screening; Community health campaigns; HPV self-collection; Implementation science; Kenya.
Conflict of interest statement
Ethics approval and consent to participate
The KEMRI Scientific and Ethics Review Unit (SERU; #2918), the Duke University Institutional Review Board (Pro0007742) and and the University of California San Francisco (UCSF) Human Research Protection Program Institutional Review Board (14–13,698) reviewed and approved the study. All participants gave their written informed consent to participate in the study prior to data or specimen collection.
Consent for publication
Not Applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Cervical cancer screening through human papillomavirus testing in community health campaigns versus health facilities in rural western Kenya.Int J Gynaecol Obstet. 2018 Apr;141(1):63-69. doi: 10.1002/ijgo.12415. Epub 2018 Jan 3. Int J Gynaecol Obstet. 2018. PMID: 29197067 Free PMC article. Clinical Trial.
-
Evaluating a community-based cervical cancer screening strategy in Western Kenya: a descriptive study.BMC Womens Health. 2018 Jul 3;18(1):116. doi: 10.1186/s12905-018-0586-0. BMC Womens Health. 2018. PMID: 29970063 Free PMC article.
-
A Qualitative Exploration of Women's Experiences with a Community Health Volunteer-Led Cervical Cancer Educational Module in Migori County, Kenya.J Cancer Educ. 2020 Feb;35(1):36-43. doi: 10.1007/s13187-018-1437-2. J Cancer Educ. 2020. PMID: 30368651 Free PMC article. Clinical Trial.
-
Optimizing high risk HPV-based primary screening for cervical cancer in low- and middle-income countries: opportunities and challenges.Minerva Ginecol. 2019 Oct;71(5):365-371. doi: 10.23736/S0026-4784.19.04468-X. Minerva Ginecol. 2019. PMID: 31698891 Review.
-
Human papillomavirus: science and technologies for the elimination of cervical cancer.Expert Opin Pharmacother. 2011 Oct;12(14):2189-204. doi: 10.1517/14656566.2011.596527. Epub 2011 Jul 15. Expert Opin Pharmacother. 2011. PMID: 21756205 Review.
Cited by
-
Systems-level barriers to treatment in a cervical cancer prevention program in Kenya: Several observational studies.PLoS One. 2020 Jul 13;15(7):e0235264. doi: 10.1371/journal.pone.0235264. eCollection 2020. PLoS One. 2020. PMID: 32658921 Free PMC article. Clinical Trial.
-
'I'm here to save my life': a qualitative study of experiences navigating a cryotherapy referral system for human papillomavirus-positive women in western Kenya.BMJ Open. 2019 Jul 24;9(7):e028669. doi: 10.1136/bmjopen-2018-028669. BMJ Open. 2019. PMID: 31345973 Free PMC article. Clinical Trial.
-
Silencing of Long Non-coding RNA RP1-93H18.6 Acts as a Tumor Suppressor in Cervical Cancer through the Blockade of the PI3K/Akt Axis.Mol Ther Nucleic Acids. 2020 Mar 6;19:304-317. doi: 10.1016/j.omtn.2019.10.041. Epub 2019 Nov 15. Mol Ther Nucleic Acids. 2020. Retraction in: Mol Ther Nucleic Acids. 2022 May 04;28:537. doi: 10.1016/j.omtn.2022.04.024. PMID: 31877407 Free PMC article. Retracted.
-
Uptake and correlates of cervical cancer screening among women attending a community-based multi-disease health campaign in Kenya.BMC Womens Health. 2022 Apr 18;22(1):122. doi: 10.1186/s12905-022-01702-4. BMC Womens Health. 2022. PMID: 35436908 Free PMC article.
-
Perspectives of women participating in a cervical cancer screening campaign with community-based HPV self-sampling in rural western Kenya: a qualitative study.BMC Womens Health. 2019 Jun 13;19(1):75. doi: 10.1186/s12905-019-0778-2. BMC Womens Health. 2019. PMID: 31196175 Free PMC article.
References
-
- Sankaranarayanan R, Swaminathan R, Jayant K, Brenner H. An overview of cancer survival in Africa, Asia, the Caribbean and central America: the case for investment in cancer health services. IARC Sci Publ. 2011;162:257–291. - PubMed
-
- World Health Organization . WHO Guidance Note. Geneva: World Health Organization; 2013. Comprehensive cervical cancer prevention and control: a healthier future for girls and women.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

