Role of endoplasmic reticulum stress in the protective effects of PPARβ/δ activation on endothelial dysfunction induced by plasma from patients with lupus
- PMID: 29208022
- PMCID: PMC5717848
- DOI: 10.1186/s13075-017-1478-7
Role of endoplasmic reticulum stress in the protective effects of PPARβ/δ activation on endothelial dysfunction induced by plasma from patients with lupus
Abstract
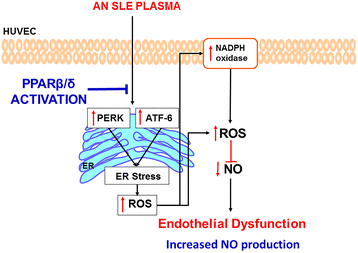
Background: We tested whether GW0742, a peroxisome proliferator-activated receptor beta/delta (PPARβ/δ) agonist, improves endothelial dysfunction induced by plasma from patients with systemic lupus erythematosus (SLE) involving the inhibition of endoplasmic reticulum (ER) stress.
Methods: A total of 12 non-pregnant women with lupus and 5 non-pregnant healthy women (controls) participated in the study. Cytokines and double-stranded DNA autoantibodies (anti-dsDNA) were tested in plasma samples. Endothelial cells, isolated from human umbilical cord veins (HUVECs), were used to measure nitric oxide (NO), intracellular reactive oxygen species (ROS) production, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, and ER stress markers.
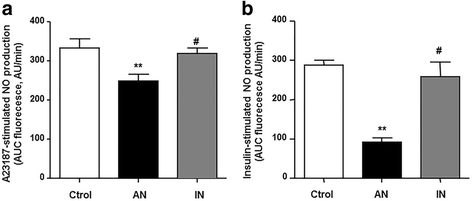
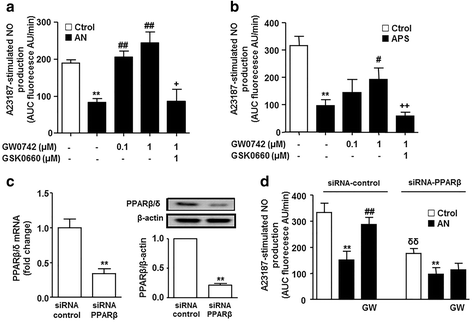
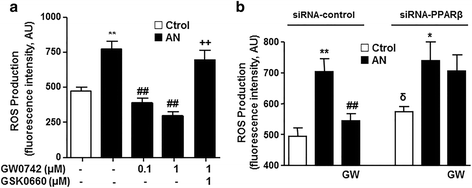
Results: Interferon-γ, interleukin-6, and interleukin-12 levels were significantly increased in plasma from patients with SLE with active nephritis (AN), as compared to both patients with SLE with inactive nephritis (IN) and the control group. The NO production stimulated by both the calcium ionophore A23187 and insulin was significantly reduced in HUVECs incubated with plasma from patients with AN-SLE as compared with the control group. Plasma from patients with IN-SLE did not modify A23187-stimulated NO production. Increased ROS production and NADPH oxidase activity were found in HUVECs incubated with plasma from patients with AN-SLE, which were suppressed by the ER stress inhibitor 4-PBA and the NADPH oxidase inhibitors, apocynin and VAS2870. GW0742 incubation restored the impaired NO production, the increased ROS levels, and the increased ER stress markers induced by plasma from patients with AN-SLE. These protective effects were abolished by the PPARβ/δ antagonist GSK0660 and by silencing PPARβ/δ.
Conclusions: PPARβ/δ activation may be an important target to control endothelial dysfunction in patients with SLE.
Keywords: Endoplasmic reticulum; Endothelial dysfunction; PPARβ/δ; Systemic lupus erythematosus.
Conflict of interest statement
Author’s information
MR is a postdoctoral fellow of the Reincorporación de Doctores del Plan Propio de Investigación, University of Granada, Spain, and MS is a postdoctoral fellow of Junta de Andalucía, Spain.
Ethics approval and consent to participate
The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The research was also approved by the Institutional Review Board Committee at the University of Granada (Reference number: 826) and all subjects gave written informed consent.
Consent for publication
All authors provided written consent to Arthritis Research & Therapy to publish this research article.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Al-Herz A, Ensworth S, Shojania K, Esdaile JM. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol. 2003;30:493–6. - PubMed
-
- Urowitz MB, Gladman D, Ibañez D, Fortin P, Sanchez-Guerrero J, Bae S, et al. Clinical manifestations and coronary artery disease risk factors at diagnosis of systemic lupus erythematosus: data from an international inception cohort. Lupus. 2007;16:731–5. doi: 10.1177/0961203307081113. - DOI - PubMed
-
- Bijl M. Endothelial activation, endothelial dysfunction and premature atherosclerosis in systemic autoimmune diseases. Neth J Med. 2003;61:273–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

