Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue
- PMID: 29208029
- PMCID: PMC5718061
- DOI: 10.1186/s13287-017-0716-x
Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue
Abstract
Background: Mesenchymal stem cells (MSCs) possess intrinsic regeneration capacity as part of the repair process in response to injury, such as fracture or other tissue injury. Bone marrow and adipose tissue are the major sources of MSCs. However, which cell type is more effective and suitable for cell therapy remains to be answered. The intrinsic molecular mechanism supporting the assertion has also been lacking.
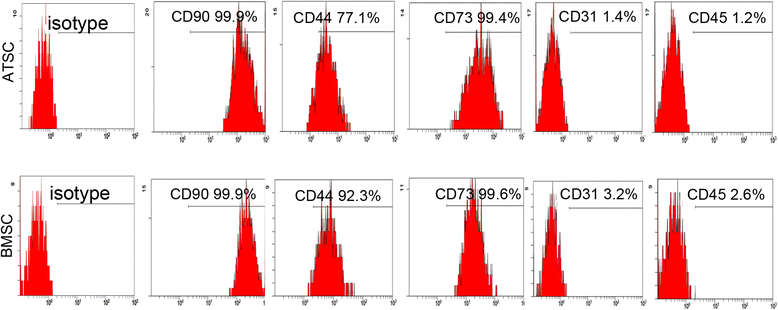
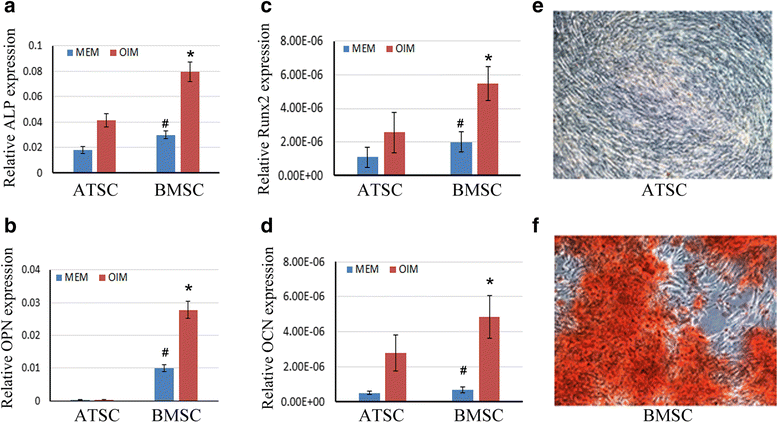
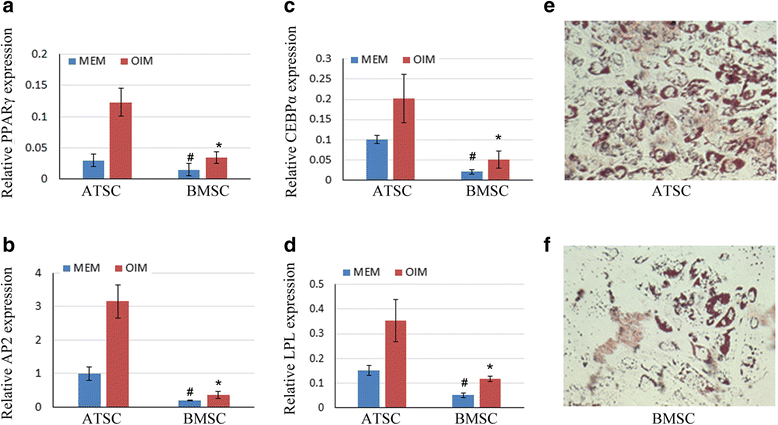
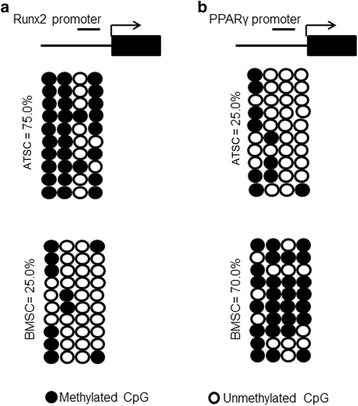
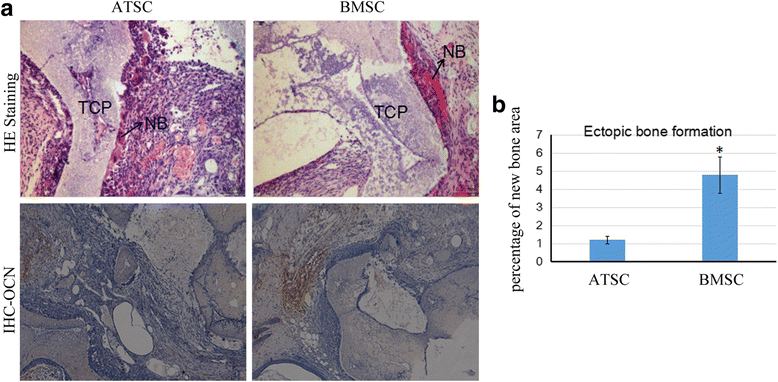
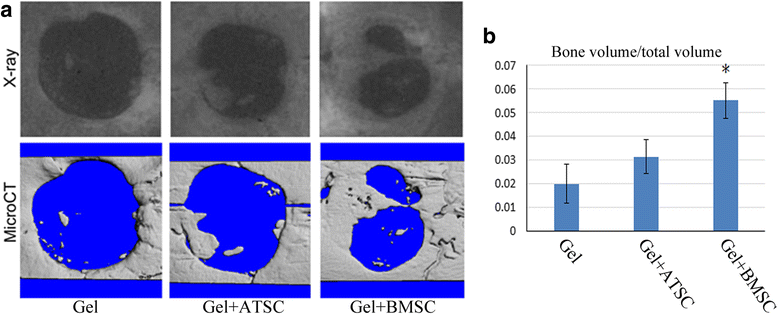
Methods: Human bone marrow-derived MSCs (BMSCs) and adipose tissue-derived MSCs (ATSCs) were isolated from bone marrow and adipose tissue obtained after total hip arthroplasty. ATSCs and BMSCs were incubated in standard growth medium. Trilineage differentiation including osteogenesis, adipogenesis, and chondrogenesis was performed by addition of relevant induction mediums. The expression levels of trilineage differentiation marker genes were evaluated by quantitative RT-PCR. The methylation status of CpG sites of Runx2, PPARγ, and Sox9 promoters were checked by bisulfite sequencing. In addition, ectopic bone formation and calvarial bone critical defect models were used to evaluate the bone regeneration ability of ATSCs and BMSCs in vivo.
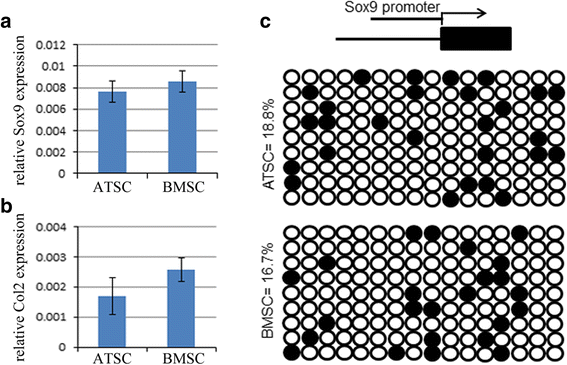
Results: The results showed that BMSCs possessed stronger osteogenic and lower adipogenic differentiation potentials compared to ATSCs. There was no significant difference in the chondrogenic differentiation potential. The CpG sites of Runx2 promoter in BMSCs were hypomethylated, while in ATSCs they were hypermethylated. The CpG sites of PPARγ promoter in ATSCs were hypomethylated, while in BMSCs they were hypermethylated. The methylation status of Sox9 promoter in BMSCs was only slightly lower than that in ATSCs.
Conclusions: The epigenetic memory obtained from either bone marrow or adipose tissue favored MSC differentiation along an osteoblastic or adipocytic lineage. The methylation status of the main transcription factors controlling MSC fate contributes to the differential differentiation capacities of different source-derived MSCs.
Keywords: Adipose tissue-derived MSCs; Bone marrow-derived MSCs; Epigenetic regulation; Mesenchymal stem cells.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
Human ethics approval was obtained from the Joint CUHK-NTEC Clinical Research Ethics Committee of the Chinese University of Hong Kong (Reference No: CRE-2011.383). Animal surgery was carried out under the animal license issued by the Hong Kong SAR Government and the approval of the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong (Ref No: 16-109-GRF). Human adipose tissue and bone marrow samples of three patients (females 60–65 years old) with total hip arthroplasty who gave informed consent were obtained from The Third Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangzhou, China).
Consent for publication
All the authors give their consents for publication in the journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Role of RHEB in Regulating Differentiation Fate of Mesenchymal Stem Cells for Cartilage and Bone Regeneration.Int J Mol Sci. 2017 Apr 24;18(4):880. doi: 10.3390/ijms18040880. Int J Mol Sci. 2017. PMID: 28441755 Free PMC article.
-
Low osteogenic differentiation potential of placenta-derived mesenchymal stromal cells correlates with low expression of the transcription factors Runx2 and Twist2.Stem Cells Dev. 2013 Nov 1;22(21):2859-72. doi: 10.1089/scd.2012.0693. Epub 2013 Jul 20. Stem Cells Dev. 2013. PMID: 23763516 Free PMC article.
-
Multilineage potential of bone-marrow-derived mesenchymal stem cell cell sheets: implications for tissue engineering.Tissue Eng Part A. 2010 Apr;16(4):1421-31. doi: 10.1089/ten.TEA.2009.0501. Tissue Eng Part A. 2010. PMID: 19951089
-
PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(3):216-25. doi: 10.2174/1574888x10666150519093429. Curr Stem Cell Res Ther. 2016. PMID: 25986621 Review.
-
Long Noncoding RNAs: New Players in the Osteogenic Differentiation of Bone Marrow- and Adipose-Derived Mesenchymal Stem Cells.Stem Cell Rev Rep. 2018 Jun;14(3):297-308. doi: 10.1007/s12015-018-9801-5. Stem Cell Rev Rep. 2018. PMID: 29464508 Review.
Cited by
-
Mesenchymal or Maintenance Stem Cell & Understanding Their Role in Osteoarthritis of the Knee Joint: A Review Article.Arch Bone Jt Surg. 2020 Sep;8(5):560-569. doi: 10.22038/abjs.2020.42536.2155. Arch Bone Jt Surg. 2020. PMID: 33088856 Free PMC article. Review.
-
Influence of platelet storage time on human platelet lysates and platelet lysate-expanded mesenchymal stromal cells for bone tissue engineering.Stem Cell Res Ther. 2020 Sep 23;11(1):351. doi: 10.1186/s13287-020-01863-9. Stem Cell Res Ther. 2020. PMID: 32962723 Free PMC article.
-
Bone marrow mesenchymal stem cells overexpressing hepatocyte growth factor ameliorate hypoxic-ischemic brain damage in neonatal rats.Transl Neurosci. 2021 Dec 17;12(1):561-572. doi: 10.1515/tnsci-2020-0204. eCollection 2021 Jan 1. Transl Neurosci. 2021. PMID: 35003786 Free PMC article.
-
Novel Potency Assay for MSC Secretome-Based Treatment of Idiopathic Male Infertility Employed Leydig Cells and Revealed Vascular Endothelial Growth Factor as a Promising Potency Marker.Int J Mol Sci. 2022 Aug 20;23(16):9414. doi: 10.3390/ijms23169414. Int J Mol Sci. 2022. PMID: 36012677 Free PMC article.
-
Osteogenic Differentiation Potential of Human Bone Marrow and Amniotic Fluid-Derived Mesenchymal Stem Cells in Vitro & in Vivo.Open Access Maced J Med Sci. 2019 Feb 14;7(4):507-515. doi: 10.3889/oamjms.2019.124. eCollection 2019 Feb 28. Open Access Maced J Med Sci. 2019. PMID: 30894903 Free PMC article.
References
-
- Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29(3):562–74. doi: 10.1111/j.1460-9568.2008.06599.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

